The study was led by Insup Choi, PhD, Assistant Professor of Neurology, and Neuroscience at Icahn Mount Sinai, with colleagues including senior author Zhenyu Yue, PhD, Aidekman Research Professor of Neurology, Professor of Neuroscience, member of The Friedman Brain Institute, Director of the Center for Parkinson’s Disease Neurobiology, and Director of the Basic Research of Movement Disorders program at Icahn Mount Sinai.

From left: Zhenyu Yue, PhD, and Insup Choi, PhD
Research has established that microglia employ autophagy when responding to amyloid plaques in brains affected by Alzheimer’s disease. Autophagy is the process by which cells self-digest and protect neurons, but aged brains have a reduced capacity for autophagy, resulting in senescence of microglia. Other recent research has implicated microglia, the brain’s resident immune cells, as critical to the development of Alzheimer’s pathology. This new study connects the dots between autophagy and microglia.
“We know from previous research that a particular subpopulation of microglia, known as disease-associated microglia, surrounds the beta-amyloid plaques in Alzheimer’s disease. Their function is to try to contain the plaques from poisoning neighboring neurons,” Dr. Yue explains. “We believe autophagy plays a very important role in sustaining the disease-associated microglia, allowing them to engage the plaques and prevent them from poisoning neighboring cells.”
Autophagy and Senescence in Disease-Associated Microglia
To look more closely at autophagy, Dr. Choi, Dr. Yue, and their colleagues turned to mouse models of Alzheimer’s disease. They found that in disease-associated microglia, autophagy was hyperactive compared to microglia from control mice. Next, they knocked out a gene essential to autophagy in the microglia. “We found that those microglia became senescent,” Dr. Choi says.
When disease-associated microglia become senescent, they lose their neuroprotective function, the study showed. But by removing senescent microglia, neurotoxicity was reduced, and normal microglia function recovered.
Senescent cells lose the power to proliferate. They also release pro- inflammatory molecules that can lead to chronic inflammation and tissue damage. Yet the role of senescence had not been well clarified in microglia, Dr. Choi says. His study showed that autophagy-deficient microglia failed to engage with beta-amyloid plaques to keep them contained. “When disease- associated microglia become senescent, they lose their neuroprotective function,” he says (see graphic below).
To learn more, the researchers sought to determine what would happen if they took those senescent microglia out of the picture. They treated autophagy- deficient mice with senolytic drugs that selectively kill senescent cells. “We found that by removing senescent microglia, neurotoxicity was reduced, and normal microglia function recovered,” Dr. Choi says.
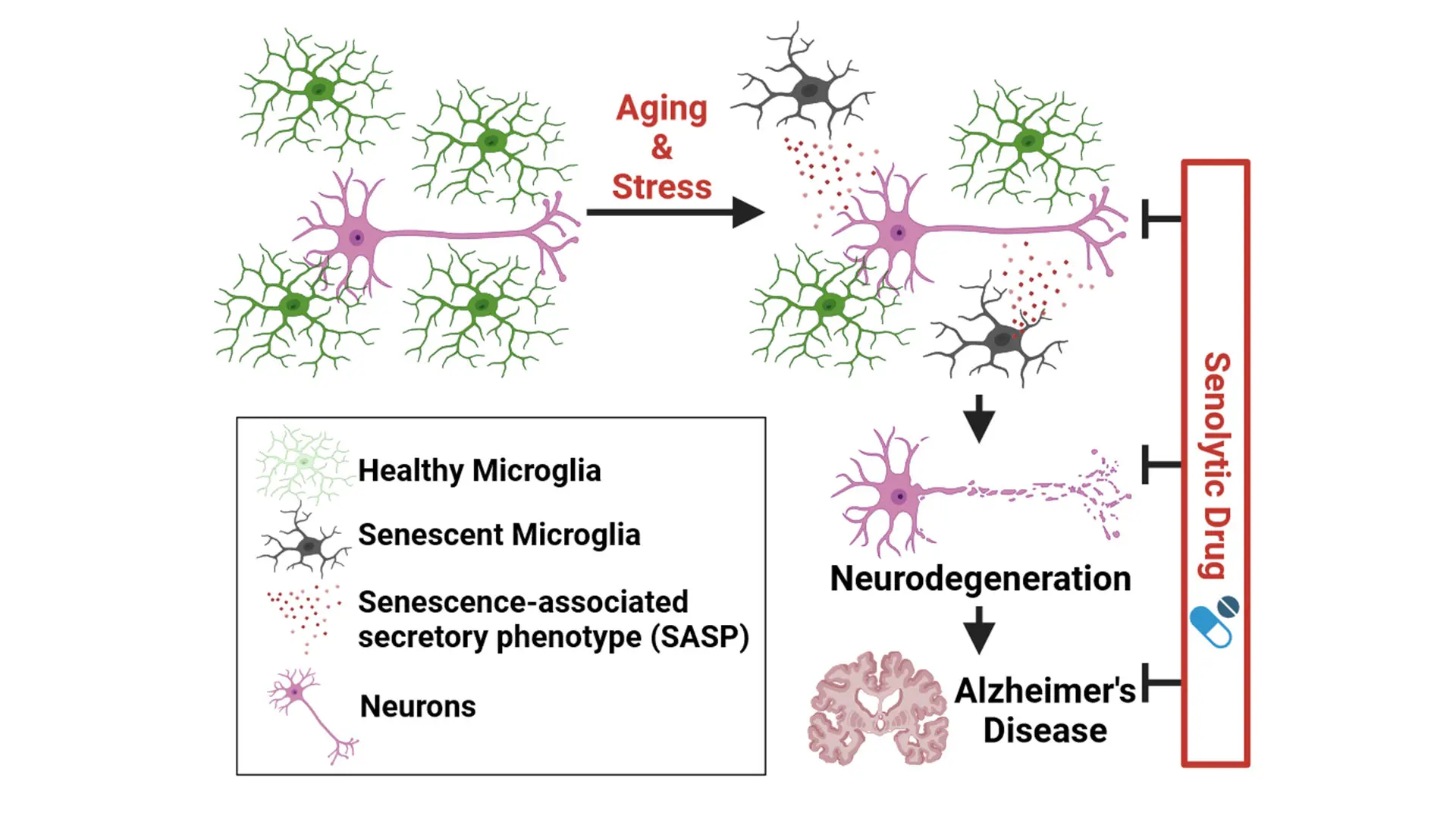
Created with BioRender.com.
This finding suggests that autophagy seems to guard microglia against senescence, enabling them to continue engaging with beta-amyloid plaques to keep them contained. “The study supports the idea that removing senescent microglia may be neuroprotective,” Dr. Choi adds.
A Treatment and Biomarker Target
Senolytic drugs are already used to target senescent cells in other diseases, though their application to the brain is relatively new. A group of scientists is conducting clinical trials to explore senolytic treatments for Alzheimer’s disease. However, such trials do not specifically target microglia, Dr. Yue says. This study suggests that zeroing in on microglia may be a promising treatment approach.
Now, he and Dr. Choi are planning next steps. Those efforts include examining human brain tissue from people with Alzheimer’s disease. “We want to confirm what we saw in animal models and understand to what degree senescent microglia may be damaging neurons in people,” Dr. Yue says.
They also plan to conduct a proteomic and transcriptomic analysis to determine the types of inflammatory molecules that senescent microglia produce—and whether those molecules might serve as targets for treatments to prevent their neuroinflammatory effects. The team also aims to explore the development of a senolytic compound specific to microglia.
This work could also lead to new ways of identifying people at risk of Alzheimer’s disease. “We lack biomarkers for diagnosing Alzheimer’s disease before symptoms appear. If this cellular senescence phenotype is strongly associated with human Alzheimer’s disease, it would be meaningful to determine whether it might be useful as a biomarker,” Dr. Choi says. “If senescent cells accumulate in the blood or cerebrospinal fluid, they could serve as a biomarker for early diagnosis.”
The findings could also inform research on other age-related neurodegenerative diseases, such as Parkinson’s disease or amyotrophic lateral sclerosis, Dr. Yue adds. “When the brain ages, microglia function declines. If we can understand the mechanism, we could potentially change the course of neurodegenerative disease—and maybe even target microglia as an antiaging regimen,” he says. “There could be broad applications of these findings in the future, and we welcome researchers to reach out to us with ideas and potential collaborations to fully explore this newly identified mechanism.”
