Zhenyu Yue, PhD, a nationally acclaimed researcher in the field, is leading the effort that is supported by the 2022 Parkinson’s Foundation Research Center Award. Dr. Yue is the Aidekman Research Professor of Neurology, Professor of Neuroscience, a member of The Friedman Brain Institute, and Director of the Basic Research of Movement Disorders program at the Icahn School of Medicine at Mount Sinai. The work will involve both the Yue Laboratory and the newly established Parkinson’s Foundation Research Center at Mount Sinai.
The new effort will allow the Yue Laboratory, in collaboration with a team of scientists led by Bin Zhang, PhD, Willard T.C. Johnson Research Professor of Neurogenetics at Icahn Mount Sinai, to continue their work to develop a comprehensive atlas of gene expression in many different cell types and characterize alterations in gene expression at single-cell type levels. “Our team will now investigate the physiological functions of the distinctive types of DA neurons and characterize the underlying molecular mechanisms of vulnerability and resilience by using three different biological systems—human postmortem tissue, genetic mouse models, and induced human DA brain cell cultures,” says Dr. Yue. “We have assembled a team of investigators whose expertise is diverse but highly integrated and complementary to one another.”

Zhenyu Yue, PhD, front center, with, clockwise, Zhuhao Wu, PhD; John F. Crary, MD, PhD; Bin Zhang, PhD; Joel Blanchard, PhD; and Nan Yang, PhD
In addition to Dr. Zhang, who is also Professor of Pharmacological Sciences, Director of the Mount Sinai Center for Transformative Disease Modeling, and a member of the Icahn Genomics Institute, the team includes:
Nan Yang, PhD, Associate Professor of Neuroscience, and a member of the Black Family Stem Cell Institute, The Friedman Brain Institute, and the Ronald M. Loeb Center for Alzheimer's Disease
John F. Crary, MD, PhD, Professor of Pathology, Molecular and Cell-Based Medicine, Professor of Neuroscience, and a member of The Friedman Brain Institute, and
Joel Blanchard, PhD, Assistant Professor of Neuroscience, and Cell, Developmental & Regenerative Biology, and a member of the Black Family Stem Cell Institute and the Ronald M. Loeb Center for Alzheimer’s Disease.
Former Assistant Professor of Cell, Developmental & Regenerative Biology at Icahn Mount Sinai, Zhuhao Wu, PhD, also remains part of the team. Dr. Wu is a principal investigator of the NIH BRAIN Initiative Cell Census Network.
“We’ve shown that the numbers of different types of DA neurons in aged human brains are significantly reduced in people with Parkinson’s disease, and so our challenge now is to demonstrate why some neurons degenerate and some survive for many years after symptom onset,” says Dr. Yue, who also serves as Director of the National Institute of Neurological Disorders and Stroke Exploratory Program for Parkinson’s Disease Research. “Once we understand the underlying mechanisms of these neuronal populations, we can start thinking about a therapeutic strategy that fundamentally alters the gene expression profile.”
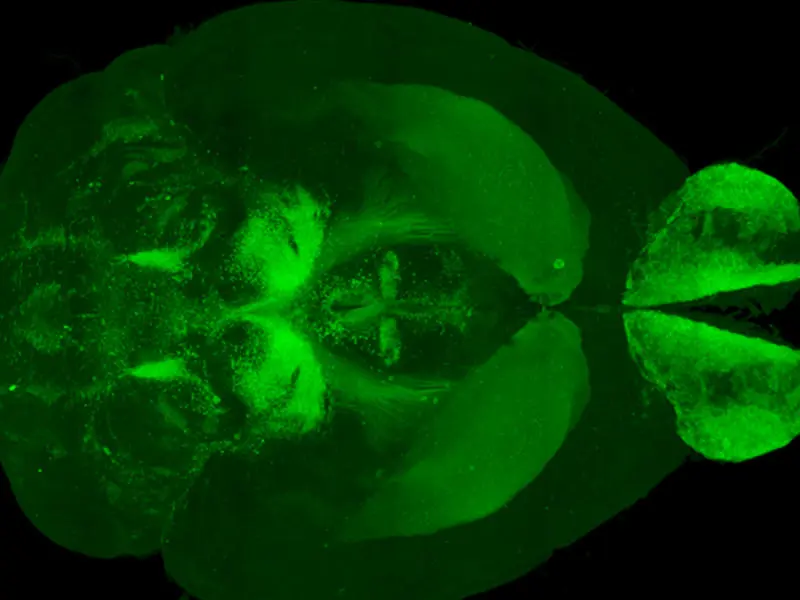
3D imaging of dopamine neuron distribution in a whole mouse brain (Courtesy of Zhuhao Wu, PhD)
Dr. Yue and his team are approaching their work with a trove of fresh knowledge, including their discovery of a unique neuron type marked by RIT2 gene expression, which is distinct from typical DA neurons and degenerate in PD brains. As part of their investigation, researchers are inducing human stem cells into DA neurons, including RIT2 and other subtypes, to better understand neuron resistance and resilience in Parkinson’s disease. Accelerating their work is the use of new technologies, such as single-cell RNA sequencing, spatial transcriptomics, and proteomics.
“Once a person develops Parkinson’s disease symptoms, like tremors, slowed movement, or fall, approximately 80 percent of the DA neurons die, but over the next 10 to 20 years, the remainder are unchanged,” explains Dr. Yue. “What our research grant will help us determine is the molecular gene transcription profile of the cells that are able to maintain their resistance.”
Central to that work is the Parkinson’s Foundation Research Center at Mount Sinai, home to the study underway. The four-year project has three components designed to probe the physiological function of the distinctive types of DA neurons and characterize mechanisms of vulnerability and resilience. The first, led by Dr. Yang, is inducing human dopaminergic brain cell cultures; the second, under the direction of neuropathologist Dr. Crary, is using human postmortem tissue from the Mount Sinai Brain Bank; and the third, led by Dr. Yue’s laboratory, is using genetic mouse models.
Providing greater impetus to the team’s work are the efforts underway globally to restore motor function to patients with Parkinson’s disease through novel cell replacement therapy and gene editing techniques. “That’s where our novel work with single-cell RNA sequencing of the entire substantia nigra is so important,” says Dr. Yue. “We want to shine light on which DA neuron or subtype is most likely to survive once it’s put back in the brain. That knowledge could help open an important new chapter in the treatment of Parkinson’s disease.”