Here, immune cells interact with a barrier layer of endfoot projections from star-shaped cells called astrocytes, which potentially provide signals to drive autoimmune attack. At the frontier of this research effort is Sam Horng, MD, PhD, Assistant Professor of Neurology at the Icahn School of Medicine at Mount Sinai, whose laboratory has discovered a novel mechanism by which astrocytes control immune cell invasion.
“Our lab is specifically interested in the contact-mediated signals exchanged between astrocytes and immune cells,” says Dr. Horng. “We’re learning how immune cells and astrocytes change their behaviors and characteristics in response to these contact- mediated interactions, and that’s a novel area that no one has looked at before.”

Senior author Sam Horng, MD, PhD, center, with postdoctoral fellow and first author Mario Amatruda, PhD, left, and associate researcher and study author Jorge Villavicencio.
In their latest research, published in Brain Communications, Dr. Horng and his team describe how they tested the hypothesis that junctional adhesion molecule-A (JAM-A), an immune cell receptor expressed by astrocytes, drives encephalitogenic T cells from within the perivascular space (PVS) into the central nervous system (CNS) to promote autoimmune demyelinating disease. Dr. Horng’s team is now exploring how this receptor affects the functional differentiation of both cell types and whether there are context- specific changes to this crosstalk in the acute stage of CNS inflammation versus a chronic stage of secondary neurodegeneration. Dr. Horng was the senior author of this multicenter study, which included investigators from seven other institutions.
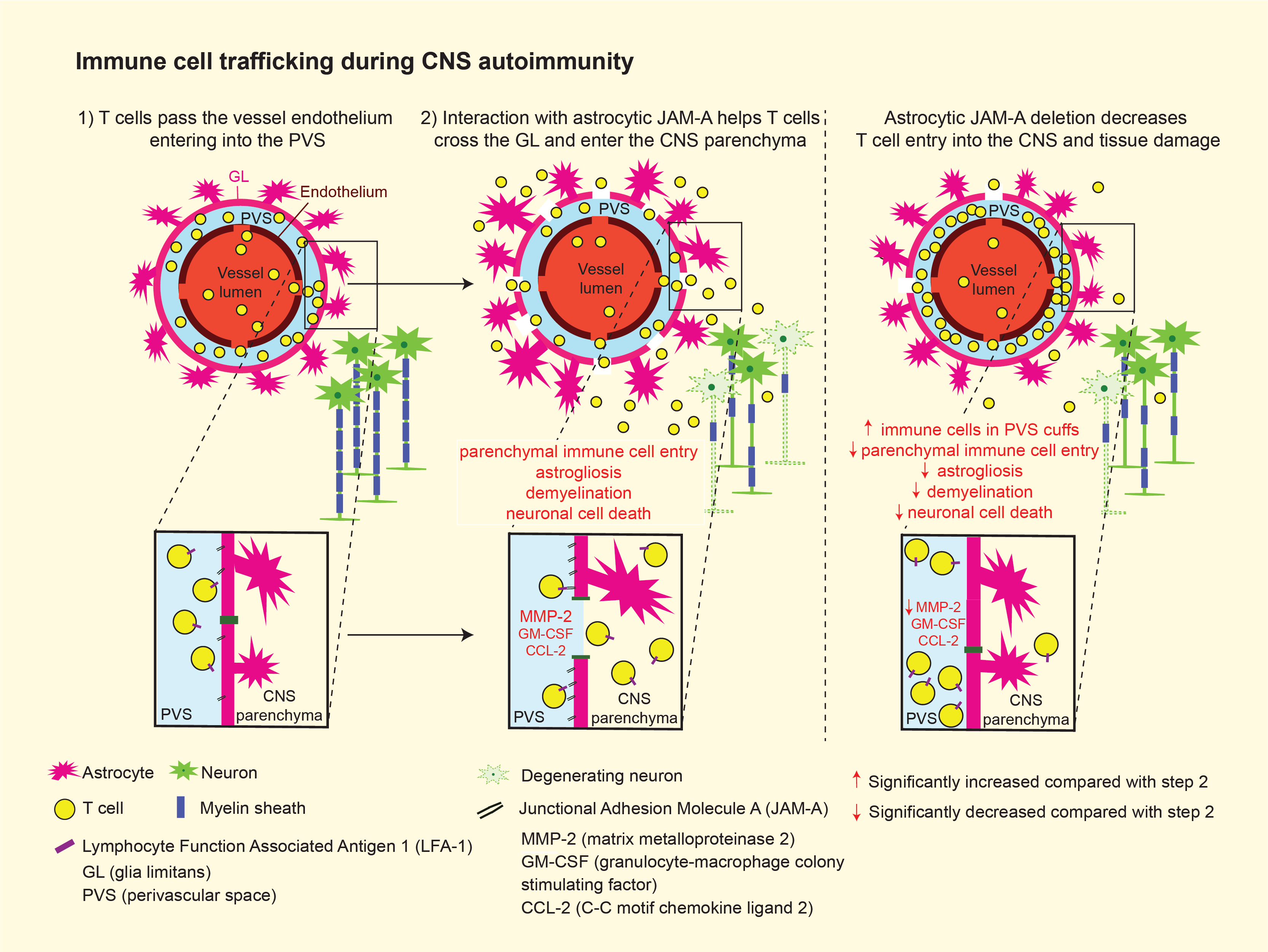
Credit: Graphical Abstract courtesy of Brain Communications
These major discoveries are segmented by the Mount Sinai-led investigators into the following four areas with the ability to significantly impact future research into multiple sclerosis and neurodegenerative disease in general:
Astrocytic immune cell receptors are upregulated in CNS inflammatory disease models
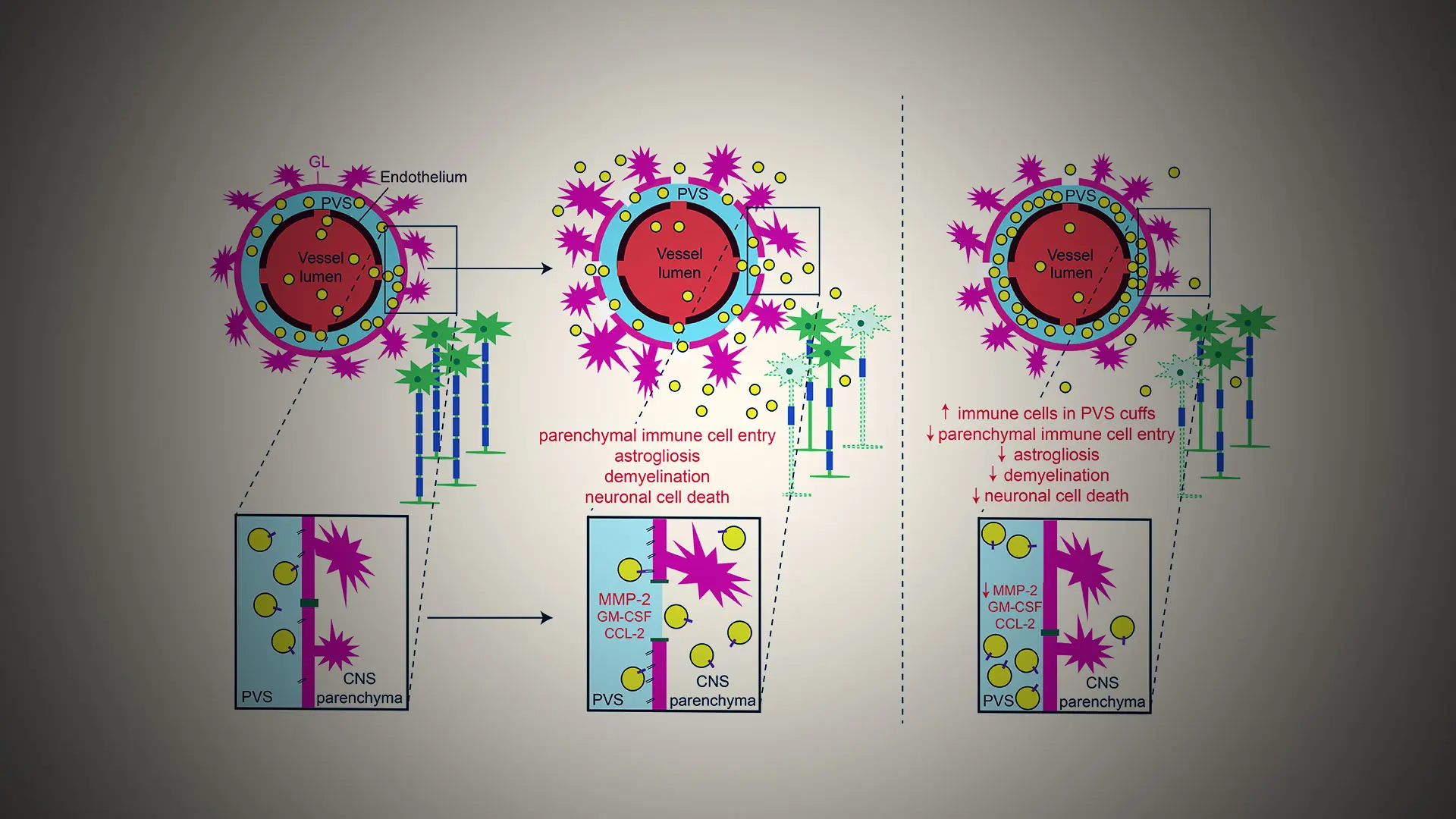
Researchers had previously learned that reactive astrocytes upregulate tight junction proteins claudin-1 and claudin-4, as well as JAM-A, an immunoglobulin-like cell surface protein that acts both in the tight junction complex and independently as an immune cell receptor. These proteins are expressed within active CNS inflammatory lesions. In this study, they identified astrocytic JAM-A upregulation diffusely on the astrocytic cell surface in both in vitro and in vivo models of CNS inflammatory disease.
“When we knocked out the JAM-A receptor in astrocytes, we found that this had a protective effect against autoimmune CNS demyelinating disease, which was unexpected because of its role in the protective tight junction complex of proteins that restricts immune cell entry,” explains Dr. Horng. “That led us to investigate its secondary role as an immune cell receptor, which led us to discover that JAM-A increases pro-inflammatory signals and promotes the entry of encephalitogenic T cells.”
Astrocytic JAM-A increases levels of cytokines and proteases critical to CNS autoinflammatory disease
In order to test how astrocytic JAM-A leads to changes in inflammatory signals, Dr. Horng’s laboratory compared the levels of a large number of proteases and cytokines in the presence and absence of JAM-A in astrocyte-immune cell co-cultures, as well as in wild- type and astrocytic JAM-A knockout mice in the spinal cords of an animal model of autoimmune demyelinating disease. What they found was that JAM-A deletion led to decreased levels of MMP-2, CCL-2, and GM-CSF—proteins involved in immune cell infiltration from the PVS into the parenchyma of brain and spinal cord tissues, and thus instrumental to pathogenic T cell activity, tissue damage, and clinical disability. “This was a particularly interesting finding,” notes Dr. Horng, “because it helped put the pieces of the puzzle together by showing that JAM-A increases levels of cytokines and proteases that had previously been identified to play a critical pathogenic role in autoimmune demyelinating disease.”
Astrocytic JAM-A regulates immune cell infiltration past the PVS
Researchers tested whether astrocytic JAM-A facilitates immune cell infiltration past the PVS and contributes to parenchymal damage by patterns of immune cell entry in two models of CNS autoinflammatory disease: asymptomatic cortical lesions and spinal cord lesions of experimental autoimmune encephalomyelitis. They learned that a higher proportion of cells co-localized in the PVS than in the parenchyma of astrocytic JAM-A knockout mice, suggesting that astrocytic JAM-A plays a role in driving immune cell entry into the parenchyma.
JAM-A deletion in astrocytes reduced tissue damage and disease severity in mice in a model of autoimmune demyelinating disease
Astrocytic JAM-A deletion decreased MMP-2 levels and T cell infiltration into the CNS of an animal model of autoimmune demyelinating disease. Astrocytic JAM-A knock-out mice also showed significantly milder courses of disease and reduced rates of mortality. An intriguing discovery by the Mount Sinai team was that systemic administration of a soluble JAM-A peptide also reduced disease severity in wild-type mice, demonstrating that blockade of astrocyte-immune cell signaling pathways from within the PVS may represent a novel therapeutic strategy against multiple sclerosis and other CNS autoimmune diseases.
“Our study identifies the therapeutic potential in understanding receptor- mediated astrocyte-immune cell interactions within the PVS,” says Dr. Horng. “The next step of our work is to identify stage-dependent effects of these interactions. How does acute versus chronic crosstalk between astrocytes and immune cells contribute to, first, the neuroinflammatory changes seen in acute relapse and then, subsequently, to the neurodegenerative changes seen in chronic disease?”