Susan Morgello, MD, Professor of Neurology, Neuroscience, and Pathology at the Icahn School of Medicine at Mount Sinai, has described in the Journal of NeuroVirology what science currently knows about the impact of COVID-19 on the central nervous system (CNS) in the context of other human coronaviruses. Here are some highlights from her review:
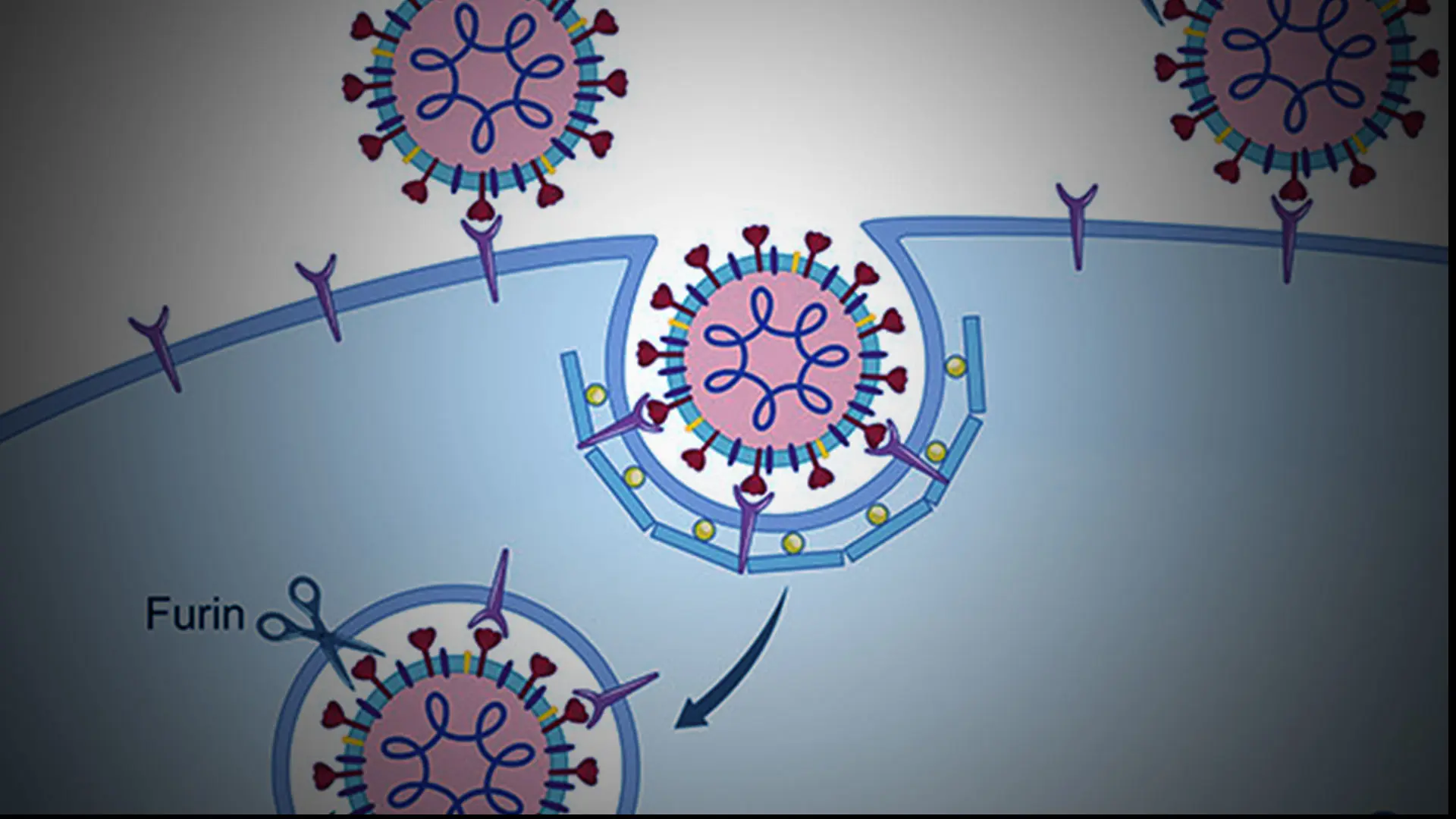
COVID-19 disease is initiated through entry of SARS-CoV-2 into human cells via the angiotensin-converting enzyme 2 (ACE2) receptor (see Figure 1). When ACE2 transgenic mice are inoculated intranasally with SARS-CoV, it spreads via transneuronal pathways into the brain, with viral protein detected in regions with first- and second-order connections to the olfactory system. Brain infection in this model is also seen with SARS-CoV-2. Symptoms related to olfactory dysfunction have been interpreted as evidence of nervous system involvement in COVID-19 but, to date, direct infection of olfactory brain regions has not been demonstrated in humans.
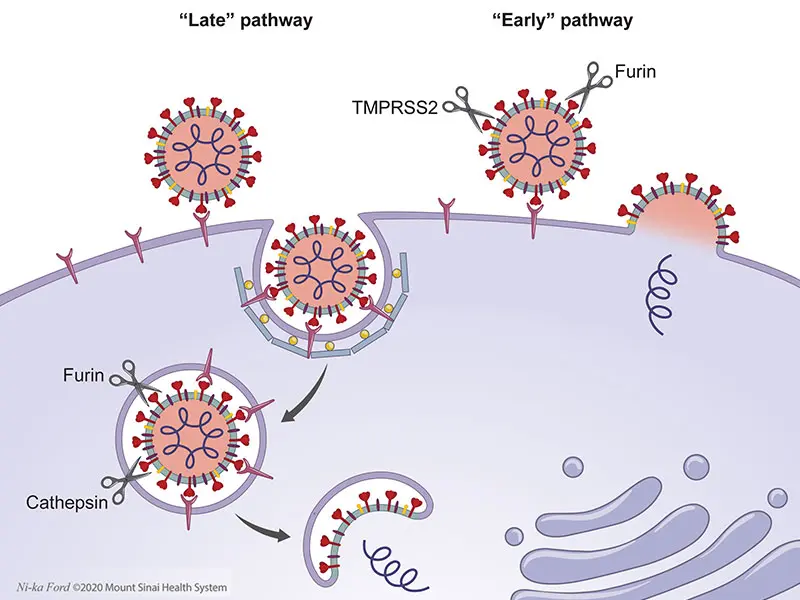
Figure 1. Cell entry pathways utilized by coronaviruses.
Above: Coronaviruses enter cell cytoplasm via two receptor-mediated pathways that require proteolytic processing of the S protein; this priming exposes the S2 domain which participates in membrane fusion and allows injection of viral genome into the cell. The “early pathway” occurs exclusively at the cell membrane, and the “late pathway” entails viral internalization via clathrin-coated pits that transition to acidified endosomes. In the late pathway, S antigen priming occurs both at the cell membrane (via proteases such as TMPRSS2 and furin), as well as in the endosome, utilizing endosomal proteases (cathepsins) and potentially furin. Therapies that interfere with acidification of the endosome, such as hydroxychloroquine, interfere with this pathway. In the early pathway, viral binding to the receptor and proteolytic processing of the S protein are accomplished entirely by proteases at the cell surface, which allows direct entry of the viral genome into cytoplasm.
The two major neurologic complications seen in infants and children with coronavirus infections are febrile seizures and acute meningoencephalitis. Febrile seizures are triggered by fever and occur without prior seizures, evidence of CNS infection, or systemic metabolic derangements. Acute meningoencephalitis is caused by mononuclear cell trafficking into the CNS. While occurring during systemic coronavirus infection, the CNS inflammatory infiltrates may or may not harbor active virus. In one study, the course of disease averaged 14.5 days and was characterized by fever, headaches, vomiting, and seizures. All children had full recoveries. Febrile seizures and meningoencephalitis are rare with SARS-CoV and SARS-CoV-2 infections. Meningitis and meningoencephalitis may be more frequent in the setting of COVID-associated pediatric multisystem inflammatory syndrome.
In adults, high rates of neurologicphenomena have been reported in those hospitalized with SARS-CoV-2, even though they are non-focal, nonspecific, and unaccompanied by direct evidence of CNS viral invasion. Based on research from Wuhan, China, abnormalities of the CNS (including dizziness, headache, and impaired consciousness) were present in 25 percent of patients; impairment of the peripheral nervous system (including taste, smell, and vision) in 9 percent of patients; and musculoskeletal system irregularities in 11 percent of patients.
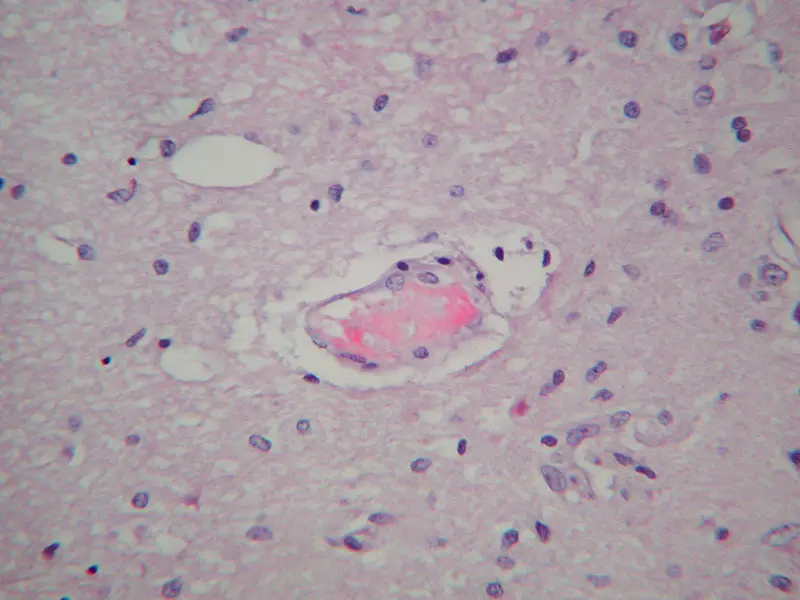
Figure 2. COVID-19-associated microangiopathy in the brain.
Above: This high-power photomicrograph displays a small blood vessel in cerebral white matter of a patient dying with COVID-19 disease. A small fibrin thrombus is seen, and monocyte margination consistent with “endotheliitis” as has been described in systemic vasculature. This patient had infarcts and hemorrhages; these findings were the most common abnormalities in the largest neuropathology series described to date (Bryce et al. 2020). (Hematoxylin and eosin stain, original magnification 200x)
A less common but well-documented complication of coronavirus is stroke, either in the form of cerebral infarction or intracranial hemorrhage. In New York City, large artery cerebral infarction was even observed in young patients with mild COVID-19 symptoms. Early in the pandemic, a manuscript by Mount Sinai researchers describing the microscopic findings in 20 brains from patients dying with COVID-19 disease (Bryce et al. 2020) was circulated in medRxiv. Widespread thrombi in microvessels with acute infarction was the most prominent finding, present in 30 percent; it is unclear if this pathology reflects the DIC-like syndrome often seen in terminal COVID-19 disease, or selective viral targeting of brain microvasculature (see Figure 2). Other findings included large parenchymal infarcts, hemorrhages, global anoxia, and, in general, sparse inflammation.
The neurobiology of SARS-CoV-2 is unknown, and both animal models and human studies will be necessary to determine whether clinical manifestations are due to direct brain infection, brain dysfunction induced by para-infectious immune and metabolic phenomena, or vascular abnormalities. This is true not only for acute disease, but also in patients with post-acute sequelae—the so-called “COVID long haulers.”
Featured

Susan Morgello, MD
Professor of Neurology, Neuroscience, and Pathology; and Chief, Division of Neuro-Infectious Diseases, Department of Neurology