Urinary albumin-to-creatinine ratio levels and estimated glomerular filtration rates (eGFR) have long been the standard for assessing patients with chronic kidney disease (CKD), specifically those with diabetic kidney disease (DKD). But Steven Coca, DO, MS, believes we are looking at the wrong end of the telescope.
"We have been focused on symptomatic management of the disease when we should be looking at which patients are more likely to experience progressive decline in kidney function,” says Dr. Coca, Associate Professor of Medicine (Nephrology) at the Icahn School of Medicine at Mount Sinai. “We have had an explosion in the number of available treatments for CKD, particularly DKD, that can halt disease progression. If we engage patients at risk much earlier than has been done historically, we will not need to talk about dialysis or transplant so much.”
To shift that conversation and prevent CKD from becoming the fifth leading cause of death worldwide by 2040, Dr. Coca co-founded Renalytix with Girish Nadkarni, MD, the Irene and Dr. Arthur M. Fishberg Professor of Medicine at Icahn Mount Sinai, and other colleagues. Launched in 2018, the company is pioneering the concept of bioprognosis™—a method of prognosis that draws on proven biomarkers, select clinical features, and machine learning to identify those patients most likely to experience progressive decline in kidney function. The company’s initial focus is on DKD, the leading cause of CKD worldwide, and that is reflected by its lead product, KidneyIntelX. This first-of-its-kind tool uses an algorithm developed by Drs. Coca and Nadkarni to assess disease progression risk among early-stage CKD patients with type 2 diabetes. The assessment is based on a variety of data inputs, including clinical data from patients’ electronic health records and three blood-based biomarkers that Dr. Coca helped validate—tumor necrosis factor receptor 1 and 2 (TNFR1 and TNFR2) and kidney injury molecule 1 (KIM-1).
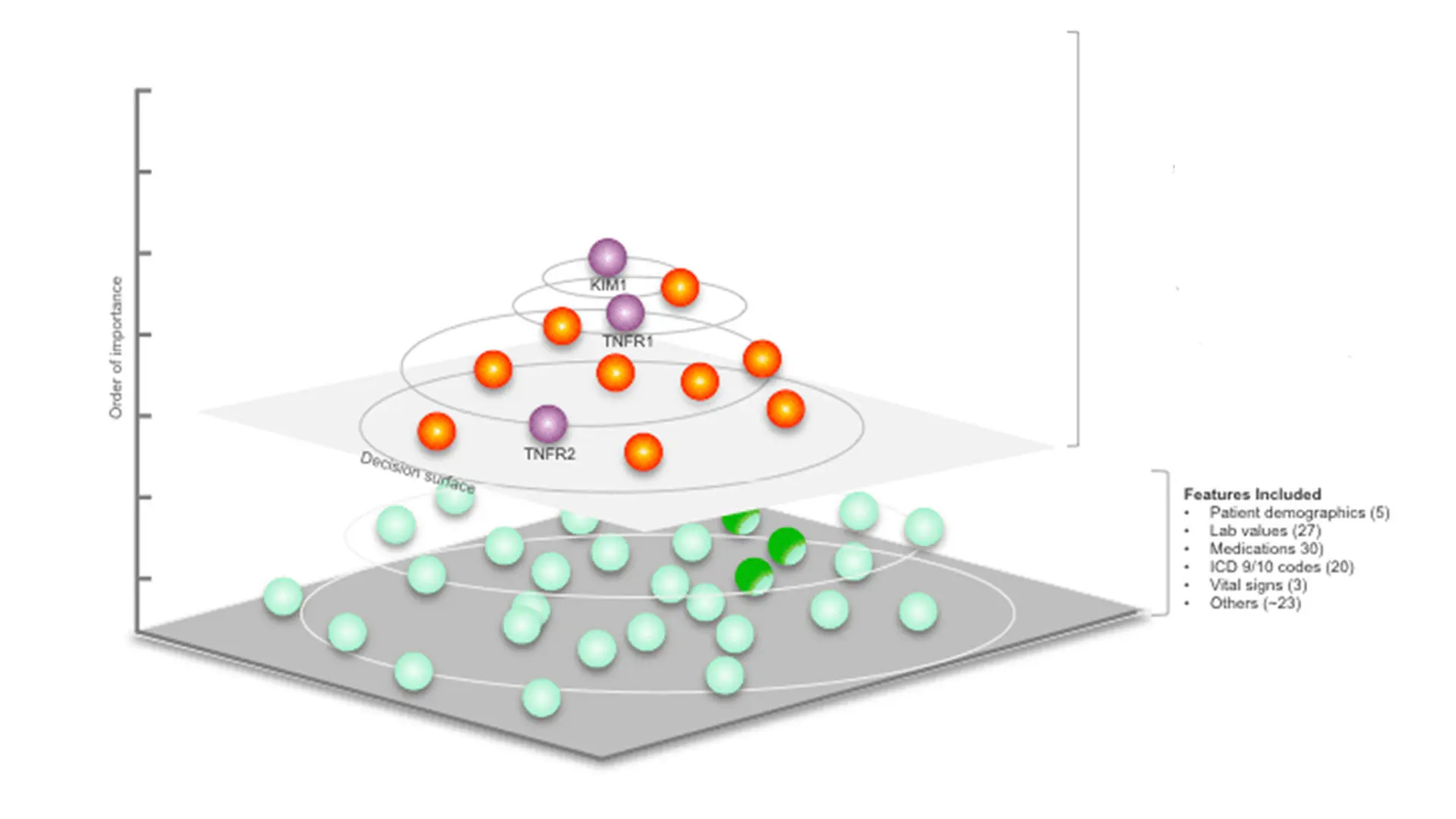
KidneyIntelX optimizes inputs from three strongly prognostic biomarkers (purple spheres) along with nine other features (orange spheres) selected from hundreds of potential features that were considered (green spheres) to generate a patient-specific score for the risk of progression of diabetic kidney disease.
“KidneyIntelX is the only commercially available prognostic test that is able to accurately identify the 15 percent to 20 percent of early-stage diabetic kidney disease patients who will experience rapid kidney disease progression, as well as those who will most likely have stable kidney function within the next five years,” Dr. Coca says. “The resulting report has three categories of risk—low, intermediate, and high—plus a numerical risk score and a recommended care pathway designed to reduce the risk of progression among patients. By targeting the approximately 90 percent of kidney disease patients who are early-stage and have no symptoms, our tool will enable earlier interventions that halt the disease from progressing to an irreversibly fibrotic state and thus ensure that patients live a long life.”
“We have been focused on symptomatic management of the disease when we should be looking at which patients are more likely to experience progressive decline in kidney function.”
- Steven Coca, DO, MS
Although the FDA indication for KidneyIntelX is for a one-time baseline risk assessment, a study led by Drs. Coca and Nadkarni using banked samples from 1,325 participants in the CANagliflozin CardioVAScular Assessment Study (CANVAS)—a landmark clinical trial assessing the benefits of administering canagliflozin, an SGLT2 inhibitor, among patients with type 2 diabetes mellitus—demonstrated the tool’s value for repeat testing. Participants chosen for the study had DKD and available baseline plasma samples. The researchers used KidneyIntelX to measure TNFR-1, TNFR-2, and KIM-1 in the samples and generated KidneyIntelX risk scores at baseline and years 1, 3, and 6 for both the CANVAS treatment and placebo groups. They then assessed the association of the baseline scores, and changes in those scores, with subsequent DKD progression.
The researchers found that KidneyIntelX successfully risk-stratified patients for progression of DKD, and they observed greater benefits on slowing the decline in long-term eGFR slope with treatment with canagliflozin versus placebo among those with higher baseline KidneyIntelX scores. Furthermore, the KidneyIntelX risk scores in the canagliflozin treatment arm declined by 5.4 percent at year one, versus a 6.3 percent increase among the placebo arm, and changes in the KidneyIntelX score from baseline to year one were associated with future risk of DKD progression independent of baseline risk score and treatment arm.
“These new analyses from the CANVAS cohort validate the prognostic performance of KidneyIntelX for progression of diabetic kidney disease,” Dr. Coca says. “In addition, these data provide the first evidence that KidneyIntelX can serve as an earlier marker of improved kidney health in response to treatment, and that changes in the KidneyIntelX score, whether higher or lower over time, are informative above and beyond the prior KidneyIntelX score.”
The path from an intriguing idea to a potentially impactful company was sometimes challenging for Dr. Coca and his colleagues, particularly the regulatory and commercial considerations necessary to launch Renalytix. He says Barbara Murphy, MD, who chaired the Department of Medicine at Icahn Mount Sinai from 2012 until April 2021, and who was a pioneer in immunology research prior to her death in June, played a crucial role in launching the company through her early support and by licensing her research. “Barbara was the first person we approached when Girish and I came up with the concept,” Dr. Coca says. “She was a tremendous champion of innovation and improvement in kidney disease and health, and she saw the value of leveraging data to do that. She secured buy-in from everyone at Mount Sinai and was really excited for the potential of Renalytix. Our work is a testament to her vision.”
Dr. Coca and his Renalytix colleagues are entering a new phase in their efforts to change the paradigm of CKD. They are planning to launch KidneyIntelX at more centers, initiating more studies to assess its efficacy in evaluating patient response to therapy, and looking to engage pharmaceutical companies in phase 4 trials of therapeutic agents to improve efficiency and uptake of new guideline-based treatments. The company has also signed a licensing agreement with the Joslin Diabetes Center for exclusive access to a comprehensive, proprietary panel of novel biomarkers, including those for kidney fibrosis, that will make KidneyIntelX an even more robust bioprognostic tool.
“Eventually, we want to add biomarkers that will expand the prognostic capabilities of the test beyond the realm of DKD,” says Dr. Nadkarni, who adds that a non-diabetic version of KidneyIntelX is in the works that will include the apolipoprotein L1 (APOL1) genotype. “In the meantime, our goal is to continue generating the necessary data to make KidneyIntelX the standard of care for risk assessment in CKD nationally and internationally.”
KidneyIntelX is based on technology developed by Mount Sinai faculty and licensed to Renalytix. Mount Sinai and Mount Sinai faculty members, including Drs. Coca and Nadkarni, have financial interests in Renalytix. Mount Sinai has representation on the Renalytix Board of Directors.
Featured

Steven Coca, DO
Associate Professor of Medicine (Nephrology)

Girish N. Nadkarni, MD, MPH
Chair of the Windreich Department of Artificial Intelligence and Human Health, Director of the Hasso Plattner Institute for Digital Health, Chief AI Officer, Mount Sinai Health System