A study by Mount Sinai has found two reproducible subtypes of polycystic ovary syndrome (PCOS) associated with novel gene regions, a discovery that could lead to improved diagnosis and to the identification of new pathways for drug targeting.
“There is increasing evidence that many common conditions, like heart failure and type 2 diabetes, are diverse collections of disease subtypes. The heterogeneity of PCOS is clinically obvious, but there have been limited attempts to specifically analyze it. We undertook this study, published in June 2020 in PLOS Medicine, to investigate whether we could identify subtypes of women with PCOS using objective mathematical approaches. Further, we wanted to confirm that subtypes we identified were biologically important by demonstrating that they were associated with different genetic signals,” says lead author Andrea Dunaif, MD, Chief of the Hilda and J. Lester Gabrilove Division of Endocrinology, Diabetes and Bone Disease, Icahn School of Medicine at Mount Sinai.
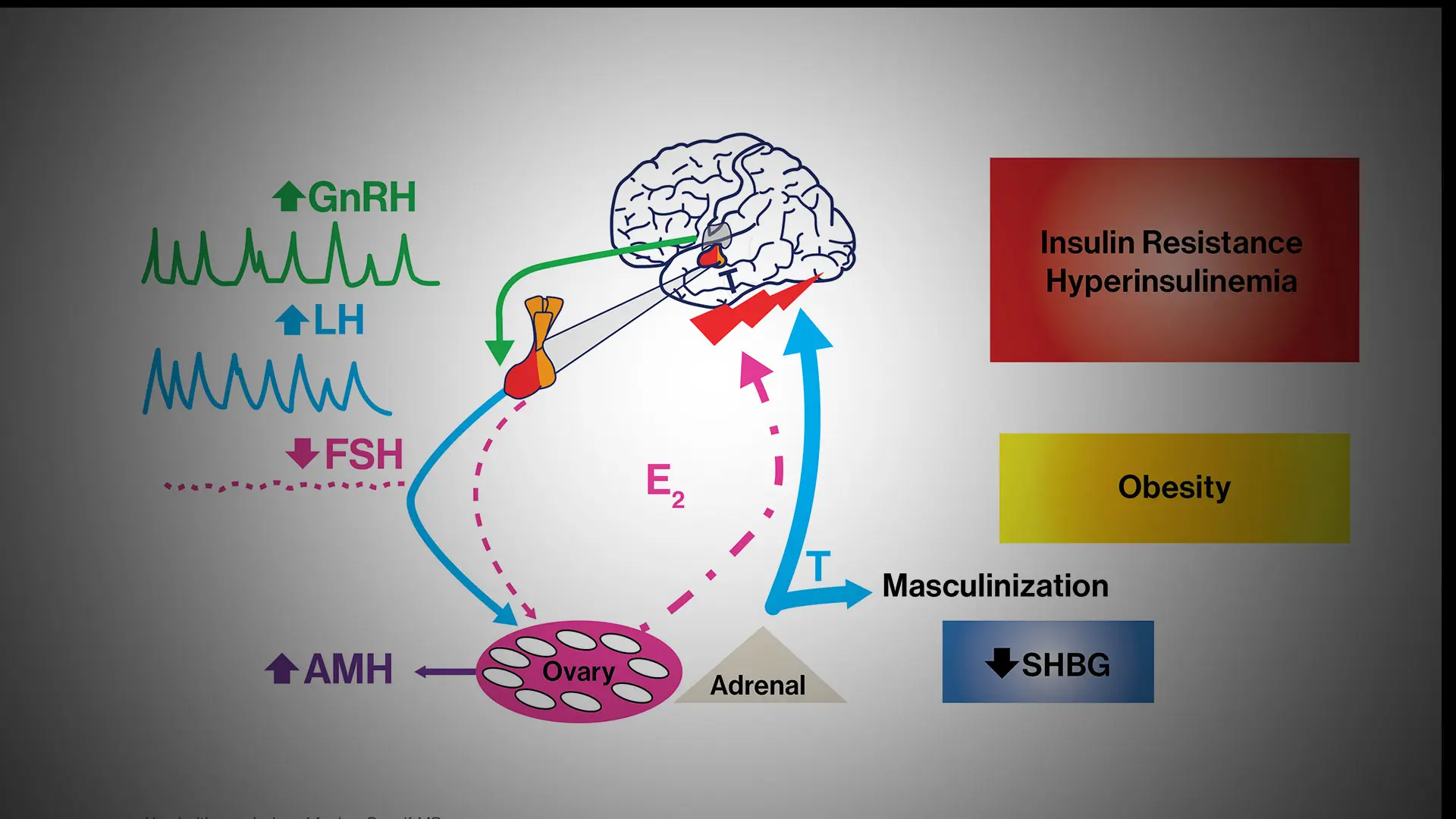
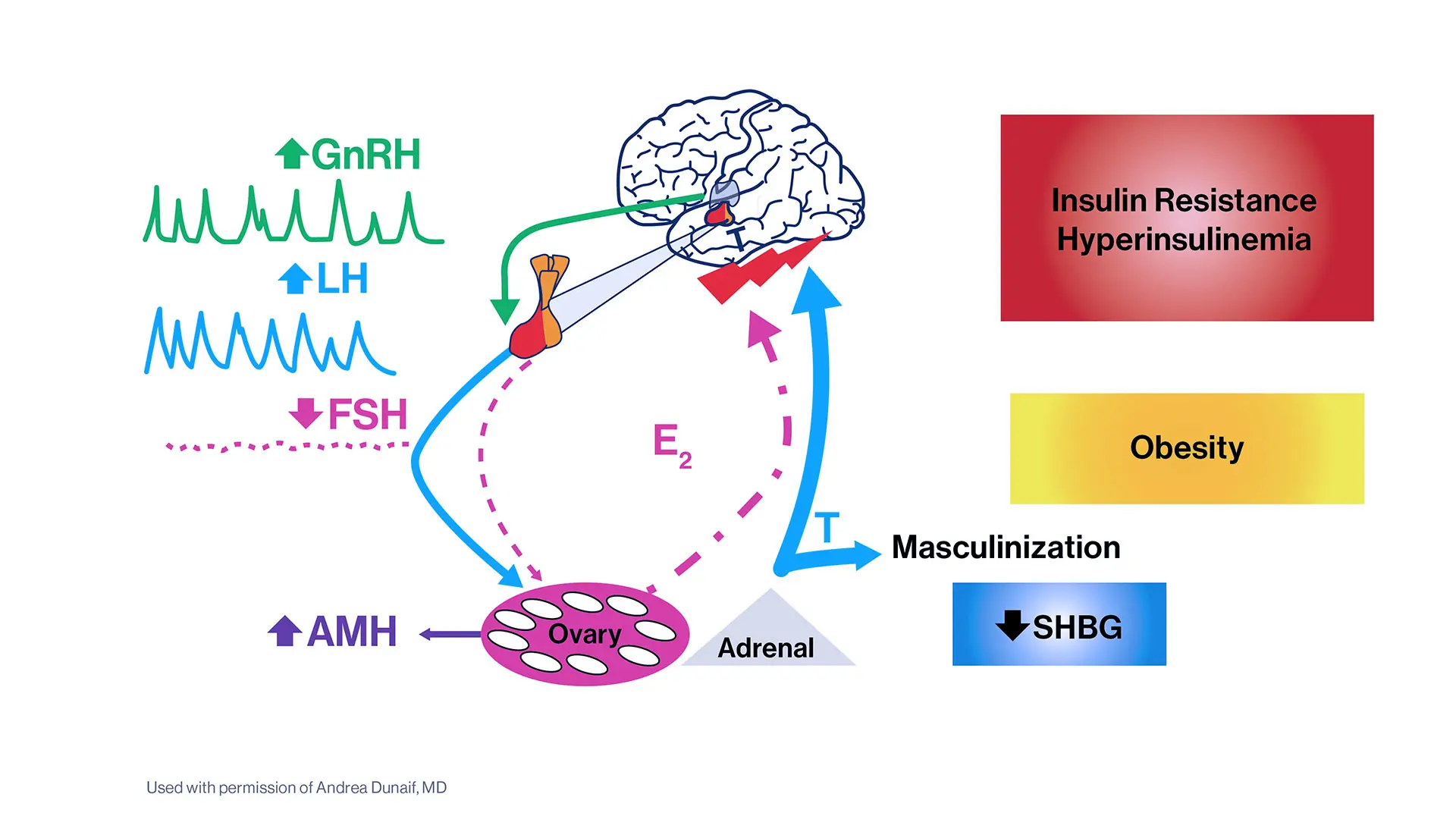
Dr. Dunaif and her colleagues used a well-established mathematical approach to aggregate hormone levels and metabolic parameters into clusters of similar data. This type of unsupervised cluster analysis makes no predetermined assumptions about the data. The clustering revealed two distinct PCOS subtypes: a “reproductive” group, characterized by higher luteinizing hormone (LH), a hormone that triggers ovulation and acts on the ovaries, and higher levels of sex hormone binding globulin (SHBG), a protein that regulates the ability of testosterone to enter its target tissues, with relatively low body mass index (BMI) and insulin levels; and a “metabolic” group characterized by higher BMI, glucose, and insulin levels, with lower SHBG and LH levels.
They then repeated a genome-wide association study (GWAS) to investigate the association between each of the PCOS subtypes and regions of the genome. They found new gene regions that were associated with each PCOS subtype. “This is a very exciting finding because the genomic loci we uncovered are strongly associated with the two subtypes and include a number of new and highly plausible PCOS candidate genes,” says Dr. Dunaif, the Lillian and Henry M. Stratton Professor of Molecular Medicine.
Dr. Dunaif, a world leader in the study of PCOS, has been investigating potential genetic components of the disorder. PCOS is the most common endocrine disorder of reproductive age women, affecting up to 15 percent of this population worldwide. It is a leading cause of infertility and type 2 diabetes. The cause, or causes, of PCOS remain unknown, so the diagnostic criteria are arbitrary and based on expert opinion rather than on knowledge of disease mechanisms.
The use of these diagnostic criteria results in three major categories of PCOS cases. However, a recent large analysis indicated that there were no significant genetic differences among these categories of PCOS cases. This finding suggests that the current diagnostic criteria do not identify biologically distinct subtypes of PCOS. In contrast, the reproductive and metabolic subtypes of PCOS were associated with biologic differences (i.e., different genes).
Characteristics of the two PCOS subtypes described in the study: the “reproductive” group, left, and the “metabolic” group.
In a previous study, Dr. Dunaif and her research team found that rare variants in a gene involved in male hormone production, DENND1A, were present in almost 50 percent of families with PCOS. It may be possible to use these genetic variants as a marker for early PCOS detection. The latest study significantly advances the investigation of the genetic heterogeneity of PCOS, Dr. Dunaif says. “To our knowledge, this is the first study to uncover PCOS subtypes with unique genetic signals that involve new gene regions.”
The gene signals associated with the PCOS subtypes, particularly those associated with the reproductive subtype, were exceptionally strong, conferring threefold to almost sixfold increases in risk, suggesting the genes causing these signals have large effects on disease risk. Signals this strong should make the genes easier to identify than typical GWAS genes, which have very weak signals. These findings need to be confirmed, but they are exceptionally intriguing and exciting, Dr. Dunaif says.
The findings could set the stage for better screening and treatment options for all women with PCOS—a disease that has been historically underdiagnosed. “Our study presents an objective approach to PCOS classification and validates this approach by demonstrating that the reproductive and metabolic subtypes thus identified are associated with novel and distinct gene regions," Dr. Dunaif says. "Further, these gene regions have large effects on PCOS disease risk, perhaps reflecting the fact that these subtypes are more homogeneous."
These strong gene signals will enable the identification of genes causing PCOS, she says, and the resulting genetic knowledge promises to uncover the causes of PCOS and identify pathways to target for innovative PCOS therapies. The strong gene signals can also be used for testing to predict PCOS. The hormonal and genetic profiles of the PCOS subtypes can be used for personalized medical approaches to PCOS.
Question of the Year:
What was the most significant development in your field in 2020?
I would say the most important development in the field was the general recognition of the importance of sex differences in disease because of the striking increases in COVID-19 mortality in men compared to women. This observation led to important studies of the mechanisms of these sex differences as well as to sex-specific therapeutic interventions.
- Andrea Dunaif, MD
Featured

Andrea Dunaif, MD
Lillian and Henry M. Stratton Professor of Molecular Medicine; Chief of the Hilda and J. Lester Gabrilove Division of Endocrinology, Diabetes and Bone Disease