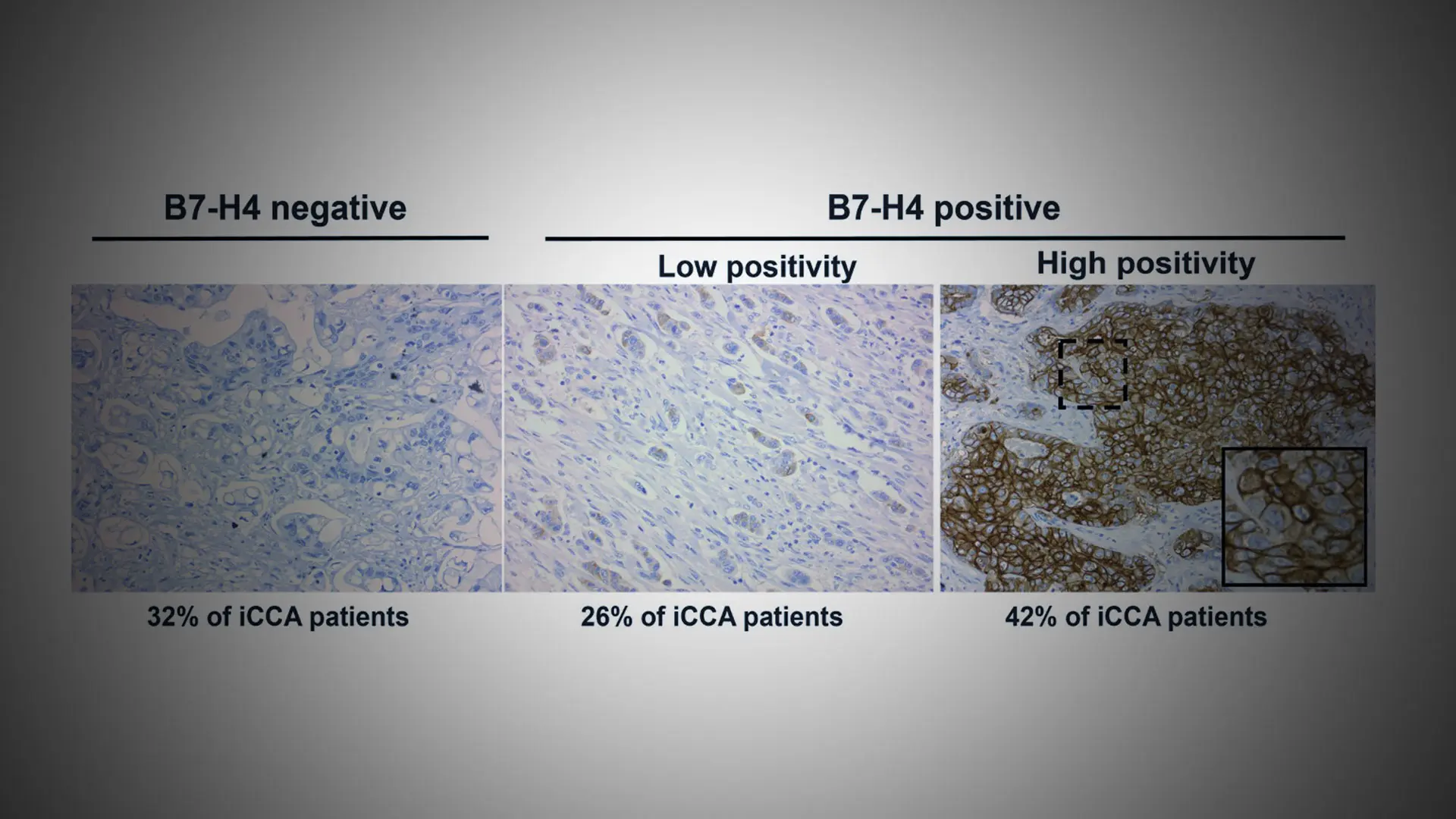
Looking at tissue samples from 80 Mount Sinai patients diagnosed with cholangiocarcinoma (bile duct cancer), Daniela Sia, PhD, noted an interesting phenomenon. Sixty percent of the samples revealed a highly elevated expression of B7-H4, a transmembrane protein that inhibits T cell immunity.
This finding was significant for Dr. Sia. In the realm of rare diseases such as cholangiocarcinoma, where breakthroughs are equally rare, the discovery of an elevated protein such as B7-H4 offers an additional potential therapeutic avenue. The current standard of care—nivolumab or durvalumab, immunotherapies that target the PD1/PD-L1 axis, combined with chemotherapy—extends survival by one month from the previous standard, which was chemotherapy alone.
“Compared with other cancers, that is not much,” says Dr. Sia, Assistant Professor of Medicine (Liver Diseases) at the Icahn School of Medicine at Mount Sinai. “However, it is significant because this new immunotherapy-based combination is the first Food and Drug Administration-approved treatment for this malignancy in 12 years. It also demonstrates how aggressive the malignancy is and how little we know in terms of treatment.”
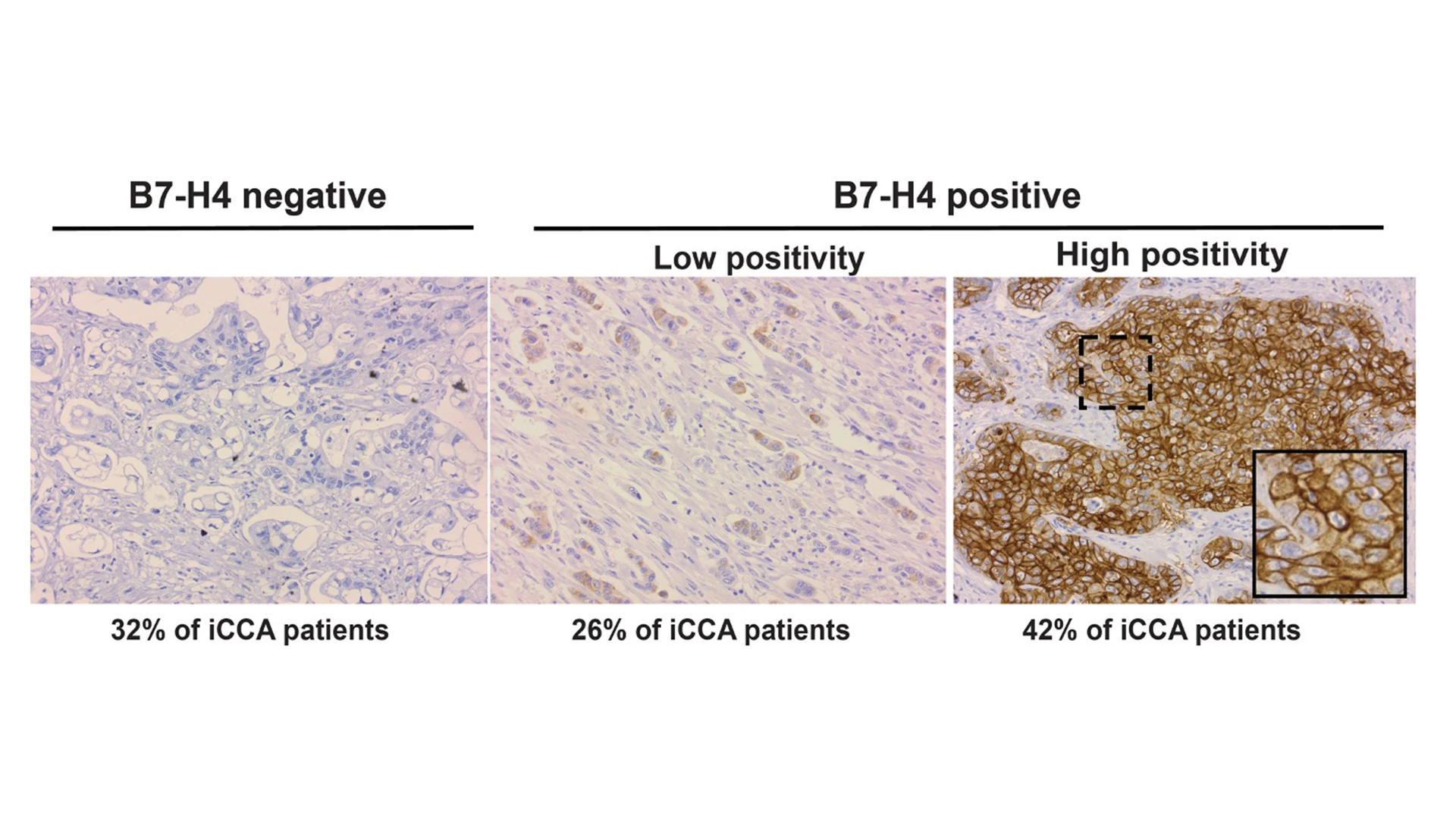
An immunostaining is used to assess the protein expression of B7-H4 in an internal cohort of cholangiocarcinoma samples. Examples of negative, low, and high positivity are shown.
The significance of these findings became clearer through in vitro analysis. Upon genetic manipulation of B7-H4 in cholangiocarcinoma cells, she observed dysregulation of transforming growth factor-beta (TGF-β) signaling and the discoidin domain receptor tyrosine kinase 2 (DDR2) gene, both of which are involved in the modeling of the stroma, a collagen-rich extracellular matrix that plays a key role in the growth of tumors such as cholangiocarcinoma. Dr. Sia and Emily Bramel, a graduate student who is focusing on cholangiocarcinoma for her thesis project, plan to submit the results for publication in early 2025.
Using Fibronest, an artificial intelligence tool that enables high-resolution, single-fiber fibrosis analysis, Dr. Sia confirmed that B7-H4 knockout mice exhibited a weaker stroma and slower tumor growth than wild-type mice. This, she notes, was unexpected for an immune checkpoint molecule.
“Typically, B7-H4 is looked at in terms of the impact on the T cells,” she says. “To find that it has an important role in stroma desmoplasia is something that is relevant not only for potentially treating cholangiocarcinoma, but also other epithelial tumors that express B7-H4.”
With these findings come new challenges. For one, the initial interaction between B7-H4 and the proteins that are crucial to direct the activation of TGF-β, which then orchestrates the deposition of the stroma, occurs intracellularly. Existing immunotherapies only block B7-H4 when it is located on the membrane of the tumor. One possible solution is to use protein degraders or oligonucleotide antisense to target B7-H4 intracellularly, but Dr. Sia says human translation of either would be a lengthy process and further delay interventions.
Ms. Bramel with Dr. Sia

Dr. Sia with assistant researcher Matias Facciuto
Another concern is that TGF-β plays a tumor-protective role in early stages of tumorigenesis and tumor-promoting role during later stages.
“For more than 15 years, investigators have tried to target TGF-β but so far it has been challenging and clinical trials have ended in failure,” Dr. Sia explains. “We are trying to look more closely at the mechanism for that upregulation to determine the other molecules and proteins that are involved. That will give us potential targets so we can block B7-H4’s impact on both the immune system and the stroma without directly touching TGF-β and possibly causing the cancer to grow much faster.”
Using single-cell data from mouse models, Dr. Sia aims to better understand the mechanisms at play in both immune response and stroma modeling to facilitate a two-pronged attack on B7-H4. She is also exploring the efficacy of novel drugs that target molecular cholangiocarcinoma mechanisms that have been uncovered with bulk RNA sequencing. Additionally, she is conducting spatial transcriptomics of cholangiocarcinoma tissue samples to identify differences, and possible therapeutic targets, among those with a high level of B7-H4 expression.
“My main focus is to find more effective therapies,” she says. “If we find promising targets through our work, we can match that with drugs that are available on the market and immediately start clinical trials.”

The cholangiocarcinoma research team includes, from left, Dr. Sia, Ms. Bramel, Mr. Facciuto, and rotating PhD student Ariel Lerner.