As the therapeutic armamentarium for Crohn’s disease continues to expand, physicians are being asked to make increasingly tough decisions about the optimal medicine for patients within the parameters of efficacy, safety, and cost. Mount Sinai researchers are helping to shed light on that process with the first head-to-head study of the biologics ustekinumab and adalimumab. In a study published in The Lancet, the team reported no significant difference in outcomes between these two major therapeutics for Crohn’s disease.
“Physicians used to be satisfied with just symptomatic relief, but with the introduction of more and more effective drugs, one of the key goals is now symptomatic remission,” says lead author Bruce Sands, MD, Dr. Burrill B. Crohn Professor of Medicine and Chief of the Division of Gastroenterology, Icahn School of Medicine at Mount Sinai. “Based on the findings of our study, they can feel confident they will get good results from prescribing either ustekinumab or adalimumab as first-line biologic treatment, provided they use them early in the disease cycle when they’re most effective.”
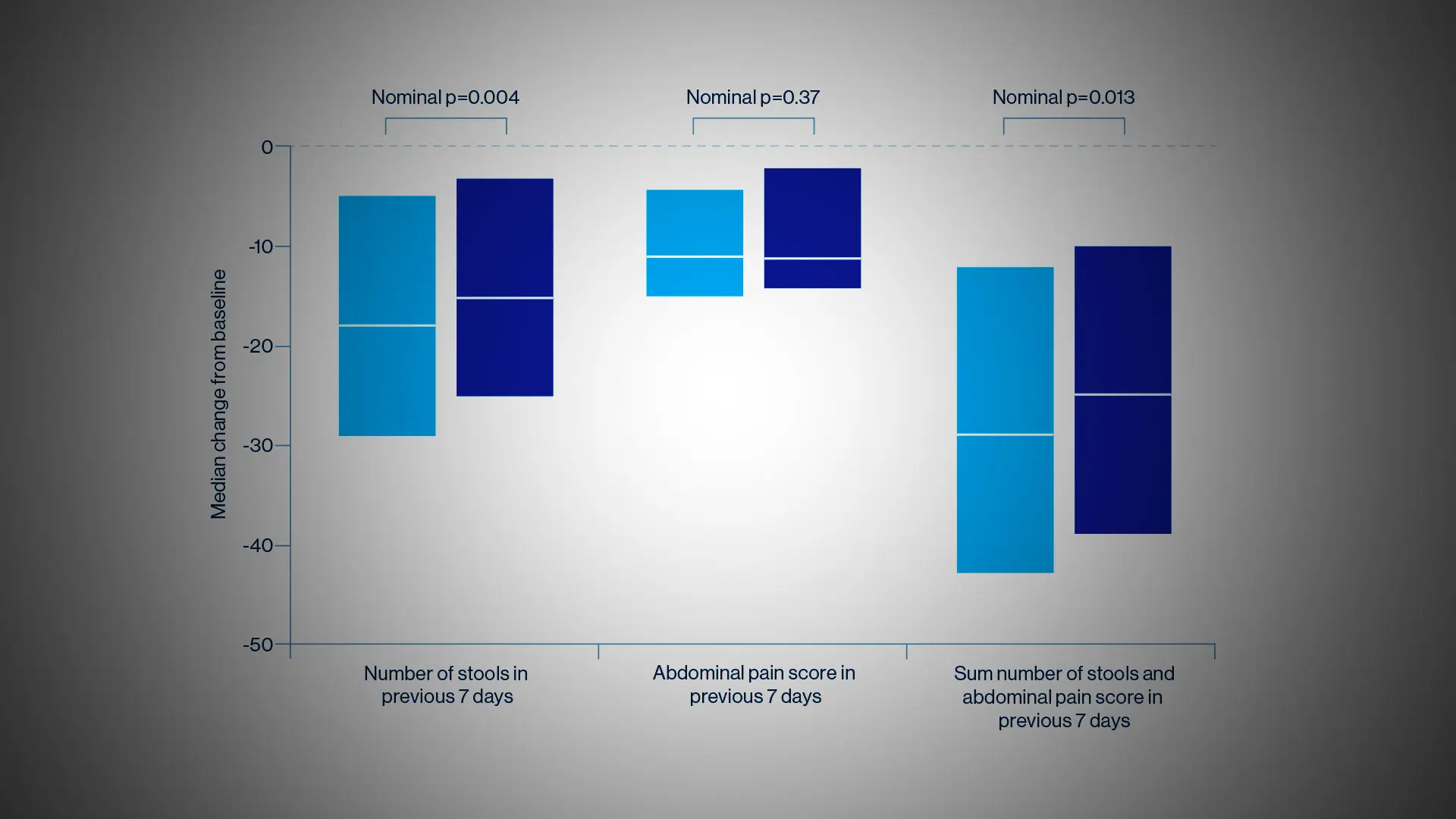
The researchers were somewhat surprised at the results since they had hypothesized the superiority of ustekinumab, a monoclonal antibody to the p40 subunit of IL-12 and IL-13, to adalimumab, a tumor necrosis factor (TNF) antagonist. But the SEAVUE study, a randomized, double-blind trial of 191 patients at 121 hospitals or private practices in 18 countries, found comparable rates of remission (nearly two-thirds of patients at the end of one year) for both biologics. This remission rate was higher than what had been anticipated for either therapy. Safety was also roughly equivalent for each, with serious infections reported in 2 percent of ustekinumab patients and 3 percent in the adalimumab group.
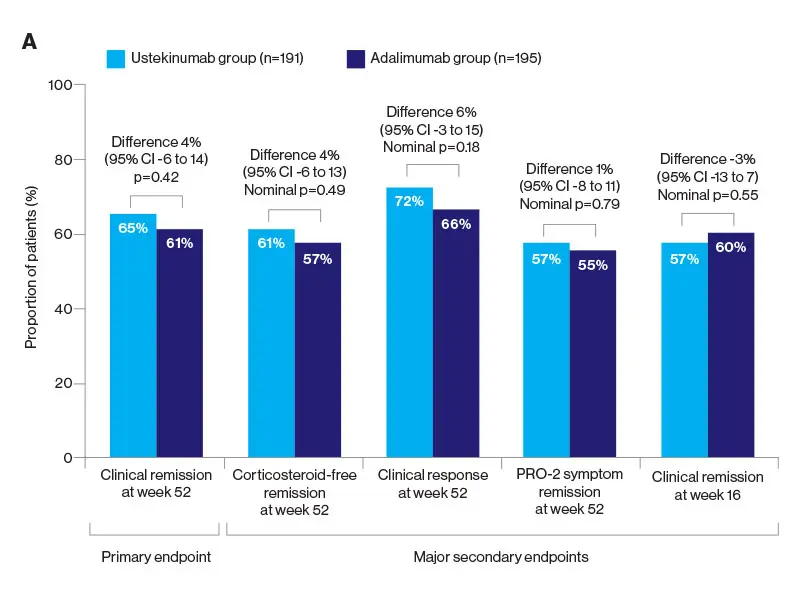
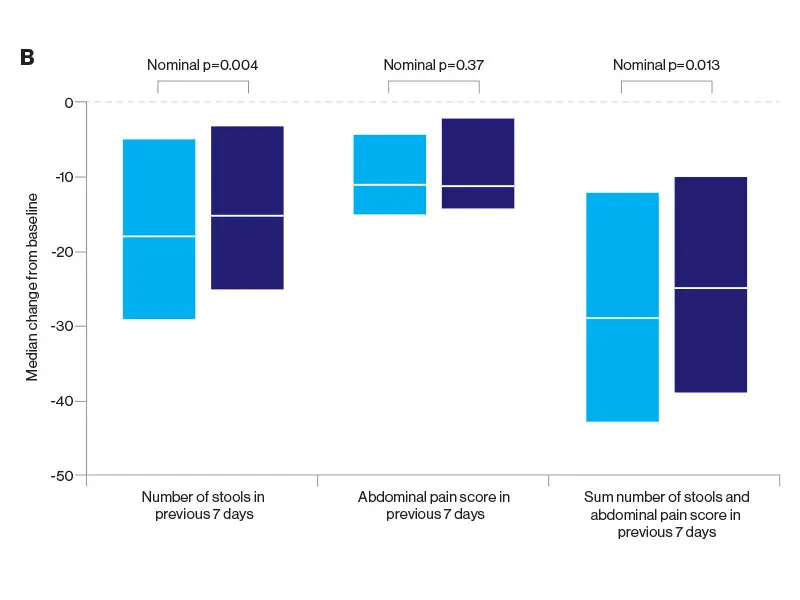
Notwithstanding those findings, physicians must weigh a host of indications and patient-specific factors before selecting one medication over the other. Decades of experience with anti-TNF drugs such as adalimumab have shown, for example, some suppression of the immune system associated with infections, as well as the risk of lymphoma and other disorders. Ustekinumab, on the other hand, is known to be quite safe, with a risk profile similar to a placebo. In terms of administration, ustekinumab involves an upfront intravenous dose followed every eight weeks by a subcutaneous injection, whereas adalimumab requires dosing every two weeks by subcutaneous injection.
“For the patient who is risk-averse or who has had prior issues with infection or perhaps a malignancy, ustekinumab will certainly be a better choice,” explains Dr. Sands, widely recognized for his innovative treatment of inflammatory bowel disease and clinical investigations of new therapeutics. “For the patient who wants a little more convenience in dosing, ustekinumab might also be a better choice. But for patients who are not concerned about those factors and must use their insurer’s mandated therapeutic, then adalimumab is an equally sound choice.”
Cost may also play a role in the selection process. While pricing of medicines is a complex issue, adalimumab is generally significantly less expensive than ustekinumab—a difference that could grow even more pronounced with the anticipated entry of multiple biosimilar adalimumabs to the market.
Dr. Sands served as Principal Investigator of another pivotal study published in 2019: VARSITY, the first head-to-head trial comparing the efficacy and safety of biologics in patients with moderate to severe ulcerative colitis (UC). That study demonstrated superior clinical remission with vedolizumab versus adalimumab at 52 weeks of treatment.
In recent years, Dr. Sands says he has witnessed a remarkable transformation in the treatment of Crohn’s disease and ulcerative colitis, fueled by biologics and the resurgence of small-molecule agents. “We’re seeing fewer and fewer patients now who have undergone multiple surgeries or who are completely debilitated by their disease,” he notes. “The availability of advanced therapies is truly making a difference.”
Featured

Bruce E. Sands, MD, MS
Dr. Burrill B. Crohn Professor of Medicine; Chief, Dr. Henry D. Janowitz Division of Gastroenterology