In a study published in Nature Microbiology, researchers at the Icahn School of Medicine at Mount Sinai address unanswered questions about how fecal microbiota transplantation (FMT) can effectively restore a patient’s microbiome.
FMT involves transferring processed stool collected from a healthy donor into the intestinal tract of a patient in order to replace the existing microorganisms in the intestinal tract and treat Clostridioides difficile infection (CDI). FMT helps to restore the balance of “good” and “bad” bacteria in the colon, which can help patients fight off infections.
“This is big news for patients and providers. While FMT is effective for CDI, it is a crude treatment, fraught with challenges, including access and safety concerns. We have identified a select group of bacteria in a real-life human study that can serve as an ideal starting point for a synthetic FMT product—without the ‘fecal’ component,” says Ari Grinspan, MD, Associate Professor of Medicine (Gastroenterology), Icahn Mount Sinai, a co-author of the study.
Studies have shown how a healthy patient’s gut microbiota after transplantation resembles that of a healthy donor. However, these studies have failed to answer a very basic question: Which individual bacterial strains are actually transferred to the recipient’s gut microbiota and for how long?
“Knowing which bacterial strains are transferred to the recipient gut microbiota is critical to our understanding of why some patients respond to FMT and others do not,” says Jeremiah Faith, PhD, Associate Professor of Medicine (Clinical Immunology), and Genetics and Genomic Sciences, corresponding author of the study.
In addition, several Food and Drug Administration advisories have reported severe adverse events from FMT, increasing safety concerns around undefined FMT, which uses the entire stool. Isolating and identifying the strains that are transferred from FMT donors to recipients could lead to a potentially safer alternative to FMT, according to the researchers.
2,987
bacterial isolates were sequenced
1,008
unique strains were represented
Dr. Faith and his team of researchers isolated and sequenced the whole genomes of 2,987 bacterial isolates representing 1,008 unique strains from 9 FMT healthy donors and 13 recurrent CDI FMT recipients, while also developing an algorithm to track sequenced bacterial strains and metagenome. The researchers identified 42 specific species that were especially likely to engraft successfully, and suggested that trials be done to ascertain which of those are most efficacious for treatment.
The researchers also rigorously validated and benchmarked the algorithms and showed that most FMT donor strains (more than 70 percent) and a minority of recipient strains (less than 25 percent) are retained for at least five years after FMT in non-relapsing patients. Patients who relapsed showed significantly less engraftment of donor strains, and within that group, those for whom a second FMT treatment was clinically successful showed significantly more engraftment after the second try. Indeed, the researchers reported that engraftment was an accurate and robust metric to explain the clinical outcome of both initial and repeat FMT.
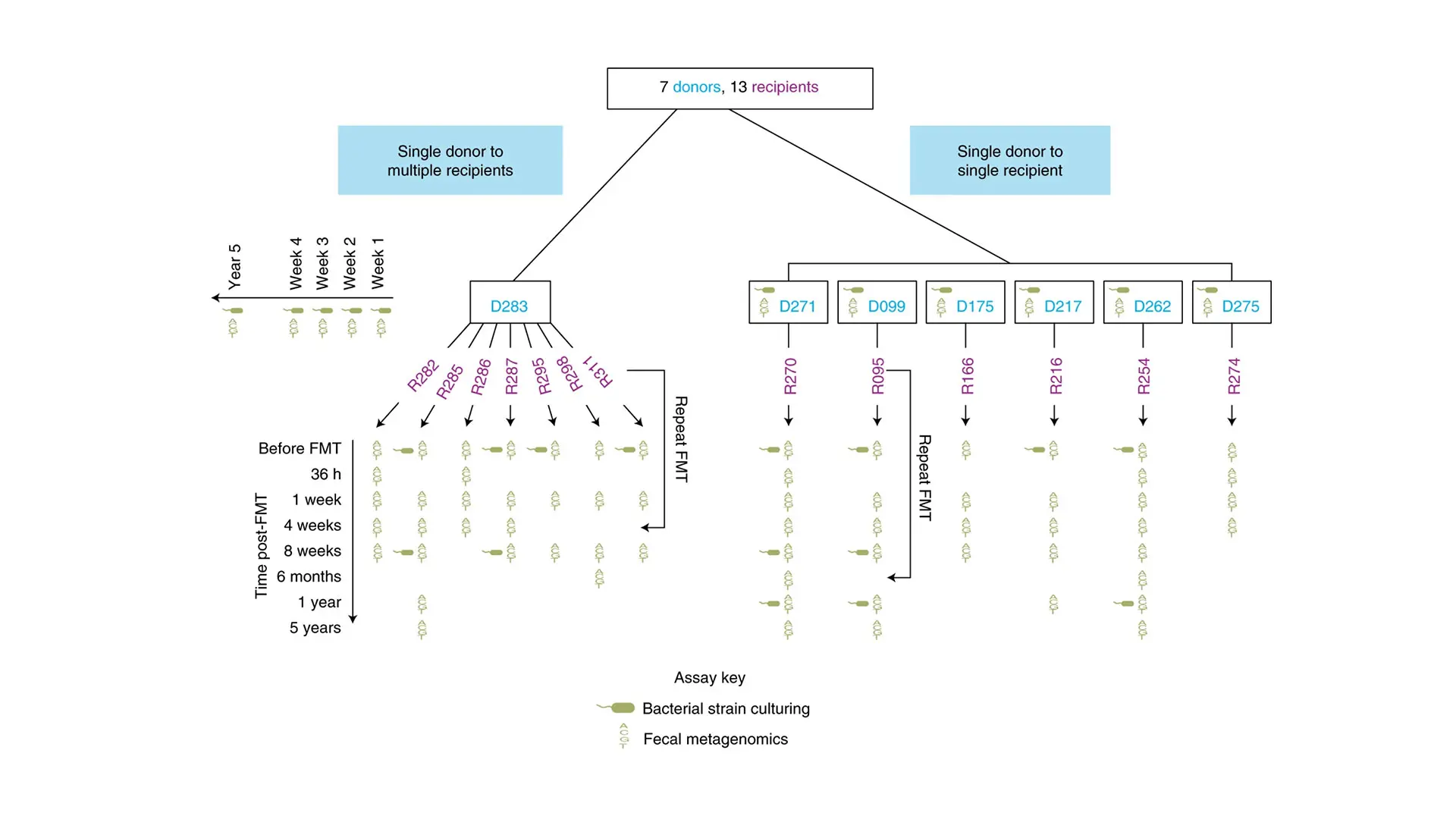
Overview of the FMT study design including donors, recipients, and time points when metagenomic sequencing and bacterial strain culturing were performed on fecal samples
Featured

Jeremiah Faith, PhD
Associate Professor of Medicine (Clinical Immunology), and Genetics and Genomic Sciences

Ari Grinspan, MD
Associate Professor of Medicine (Gastroenterology)
