Unsatisfied with the current model of invasive biopsies, imprecise ultrasounds, and all-too-often late diagnosis and poor prognosis for patients with hepatocellular carcinoma (HCC), Mount Sinai researcher Augusto Villanueva Rodriguez, MD, PhD, is looking to revolutionize it.
“Liver cancer has become the second most lethal cancer after pancreatic cancer,” says Dr. Villanueva, Assistant Professor of Medicine (Hematology and Medical Oncology, and Liver Diseases), at the Icahn School of Medicine at Mount Sinai. “It has also had the highest increase in mortality in the last 10 years, so there is an acute need for more precise tools that enable early interventions and more informed decisions regarding treatment. I believe liquid biopsy and intratumoral heterogeneity (ITH) hold the potential to identify the biomarkers and drivers of HCC tumor development and treatment resistance.”
Although researchers have known since the 1950s that fragments of tumor DNA can circulate in the blood, the potential oncological applications of liquid biopsy have only emerged in recent years as advances in technology have enabled researchers such as Dr. Villanueva to conduct imaging flow cytometry, next-generation sequencing, and droplet polymerase chain reaction on tumor cells, DNA, and extracellular vesicles (EVs). “Ultrasound with alpha fetoprotein, which is what we use now to detect early stage tumors, has a 63 percent sensitivity, which is very poor,” Dr. Villanueva observes. “With liquid biopsy, available data suggest we can achieve 85 percent sensitivity or higher, which will give us a more precise tool for early interventions.”
Dr. Villanueva has made some progress in identifying possible prognostic tools using liquid biopsy. He conducted a study, published on bioRxiv, to assess the role of EVs in HCC in tumor initiation and metastasis—focusing on unannotated genomic regions, which he believes hold promise as biomarkers for early cancer detection. Working with his lab, Dr. Villanueva identified small RNA clusters (smRC) and delineated their key genomic features across biospecimens (i.e., blood, urine, tissue) and EV isolation techniques.
A 3-smRC signature for early HCC detection was trained and validated in two independent cohorts: 100 patients with early stage liver cancer and 100 controls. The team noted that a 3-smRC signature was significantly overexpressed in circulating EVs of HCC patients compared to at-risk controls or patients with non-HCC malignancies (p<0.01, n=157). A phase 2 biomarker study to validate these findings revealed 86 percent sensitivity and 91 percent specificity for the detection of early HCC from controls at risk (i.e., those with cirrhosis or chronic liver disease).
“These findings are promising, but we need to test them using a larger set of samples to confirm if this biomarker could be deployed in clinical practice,” Dr. Villanueva says.
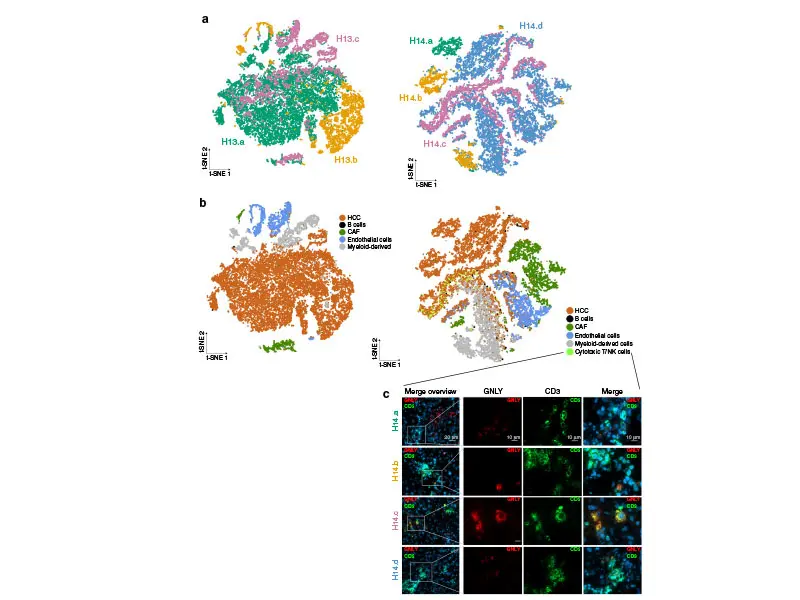
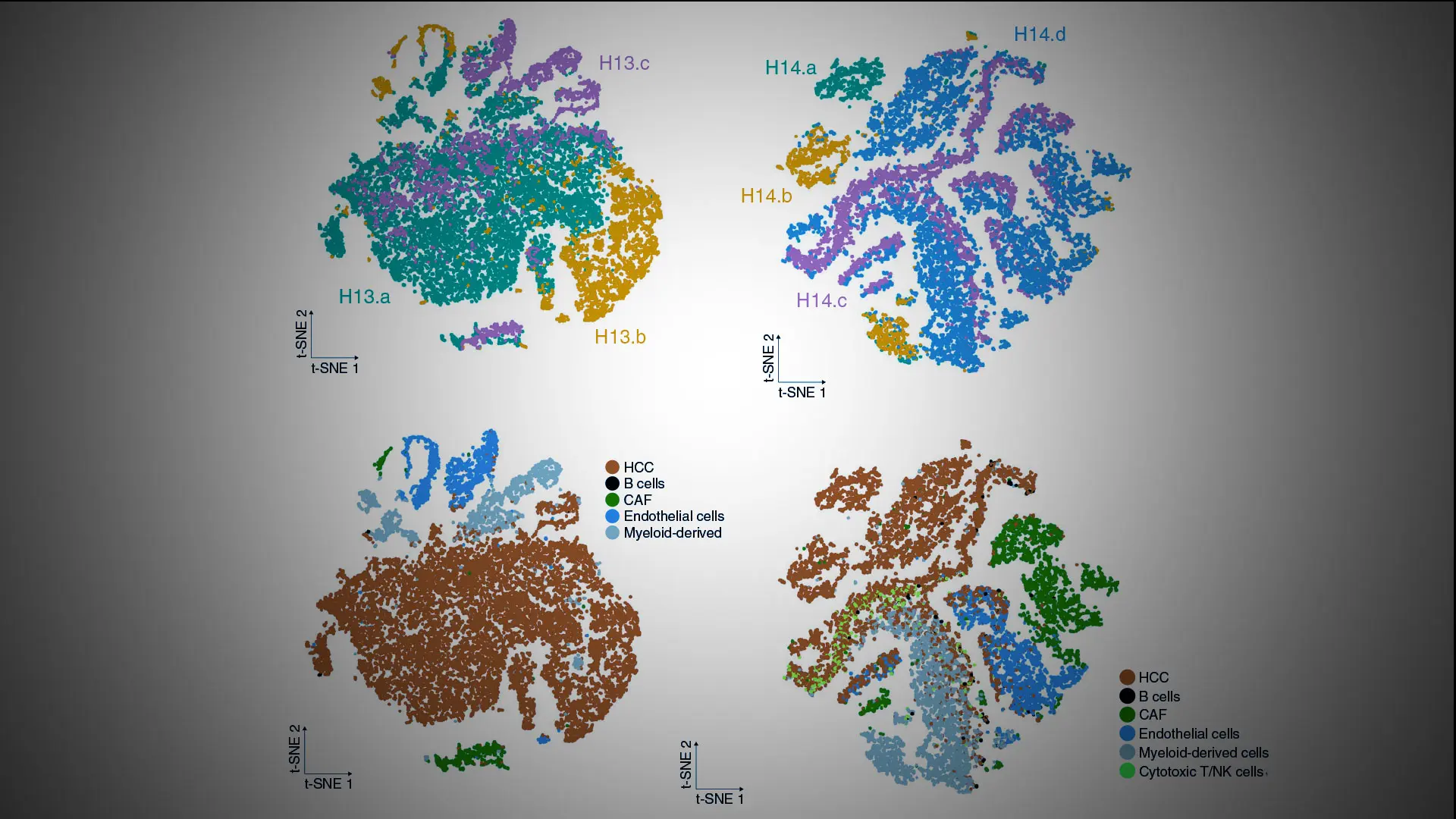
HCC ecosystem and regional transcriptomic heterogeneity on single-cell RNA-seq. Reprint from "Intratumoral heterogeneity and clonal evolution in liver cancer," Nature Communications
Dr. Villanueva’s research involving intratumoral heterogeneity and associated biomarkers and drivers is also providing new insights into HCC evolution and treatment resistance. Using a dataset of 71 multiregional samples—51 tumoral and 20 nontumoral adjacent regions—from 14 HCC patients who were treated with surgical resection, he conducted the largest, most comprehensive analysis of transcriptomic ITH in HCC reported to date. The research team observed ITH in 30 to 40 percent of treatment-naïve HCC encompassing neoepitope burden, tumor-infiltrating-lymphocyte burden and clonality, hepatitis B virus expression, and regional gene expression. The study, “Intratumoral heterogeneity and clonal evolution in liver cancer,” was published January 15, 2020, in Nature Communications.
“These findings are significant in that they show that a biopsy taken from the same tumor can reveal different molecular aberrations depending on where it is taken, which means you can have opposing information on whether a particular therapy is likely to be effective for a patient,” Dr. Villanueva says.
The study is also groundbreaking in that it is one of the first to assess the role of the immune system in the evolution of liver cancer. Dr. Villanueva noted significant regional differences in the magnitude of immune cell infiltration, suggesting that tumor-immune interactions contribute to the emergence of ITH.
“In some regions, we saw heavy infiltration of immune cells while in other regions of the same tumor nodule we observed barely any infiltration at all,” he explains. “If we can identify the mechanism that drives this phenomenon, it may provide us with a means of increasing this infiltration and thus achieving a higher response rate to immune-based therapies, and we will be exploring that in a follow-up study with a larger set of samples.”
Featured

Augusto Villanueva Rodriguez, MD, PhD
Associate Professor of Medicine (Hematology and Medical Oncology, and Liver Diseases)

Bachir Taouli, MD
Professor of Diagnostic, Molecular and Interventional Radiology