Moving towards precision medicine for improved patient care is a shared mission for Mount Sinai researchers.
Precision medicine, which takes into account each patient’s genetics, environment, and lifestyle, represents a paradigm shift away from the “one-size-fits-all” approach to healthcare. Instead of defaulting to guidelines developed for the average person, clinicians see each person for who they are — a unique individual who needs disease treatment and prevention strategies tailored for their disease.
“One of the big challenges in medicine is that, in many cases, we’re trying to implement one therapy across many different patients.”says Anna Tocheva, PhD, Assistant Professor of Genetics and Genomic Sciences at the Icahn School of Medicine at Mount Sinai. “It’s a collective effort to improve patient health at Mount Sinai, and for the Department of Genetics and Genomic Sciences, personalized and precision medicine is a key focus.”
Researchers at Mount Sinai are actively working toward approaches that will allow doctors to predict more accurately which treatment and prevention strategies for a particular disease will work best for a given patient. While such methods are practiced in some cases, expanding precision medicine has the potential to improve patient care and outcomes in all areas of healthcare.
For example, Tocheva and her colleagues develop miniature 3D models of patient tumors in the lab to get a personalized view of their responses to therapy. These 3D models, known as patient-derived tumor organoids, better capture the unique genetic makeup of each patient’s tumor unlike animal models and are able to predict patient responses to therapy. Specifically, the researchers pair the patient-derived organoids with immune cells taken directly from the tumor. This approach allows them to test patient-specific responses to cancer immunotherapies that boost immune cell activity, but also to understand why some patients respond to immunotherapies while others do not.
“Now that we have access to this fantastic personalized approach, our goal is to use this at scale, in high throughput, to identify the optimal treatment for each unique patient.”
She estimates that generating the organoids and obtaining preliminary data on different therapies will take about four weeks, which fits into the timeline for clinicians to decide on a treatment strategy.



The lab of Bin Zhang has developed a highly efficient pipeline for repurposing “old” drugs for new indications. Drug repurposing, a strategy for identifying new therapeutic uses for existing drugs, has several advantages over developing a new drug from scratch. Existing drugs approved by the Food and Drug Administration (FDA) have already been deemed safe for human use, meaning they can offer a more efficient, cost-effective approach to drug discovery.
Zhang’s pipeline works by quantifying the capability of an existing drug in normalizing the molecular dysfunctions that cause disease. This approach leverages artificial intelligence (AI)- and machine learning based methods to associate drug effects with disease signatures derived from genomics, transcriptomics, proteomics, metabolomics, and clinical data from individual patients.
“We are moving very aggressively towards this kind of precision medicine and have already applied this pipeline to a number of complex human diseases, including Alzheimer’s disease, 32 types of cancers, obesity, and diabetes,” says Zhang, PhD, Willard T.C. Johnson Research Professor of Neurogenetics in the Department of Genetics and Genomic Sciences. “Also, we are working with our colleagues in the Department to find therapeutics for patients with rare genetic disorders.”



Zhang, who also serves as Director of the Mount Sinai Center for Transformative Disease Modeling, then uses virtual clinical trials to further evaluate the performance of a repurposed drug for the unintended condition. His lab incorporates real patient data from large-scale repositories like Mount Sinai’s BioMe and UK Biobank to find correlations of a specific drug with unintended patient outcomes.
For example, a certain drug may be able to improve cognitive performance as an unintended side effect that proves beneficial to patients. With a virtual clinical trial, Zhang and his colleagues can determine whether a group of patients who took the drug over, say, five years experienced a significant boost in cognitive performance versus a control group.
“Real clinical trials at such a large scale require a significant amount of money and time, whereas we can survey millions of patients in one or two days with a virtual clinical trial,” says Zhang.
“Together with the drug repurposing pipeline, virtual clinical trials will give us a great advantage for making contributions to precision medicine.”
Today’s researchers and clinicians have a wealth of technologies that monitor a patient’s immune system at their fingertips, from whole-genome and RNA-sequencing to microbiome analysis and immune cell profiling. But the explosion of information has highlighted the need for better ways to interact, explore, and visualize such datasets.
To this end, the Gümüş Lab focuses on the development of novel data visualization and analysis techniques that can help identify drivers of disease and drug targets for precision medicine. For example, PRIMAVO (Precision Immune Monitoring Assay Visualization Online) is a user-friendly tool for better understanding the mechanisms behind cancer immunotherapy. Since cancer immunotherapy only works for a minority of patients, with others experiencing adverse side effects, the hope is that PRIMAVO can empower researchers to understand which patients will benefit and why.
“Not everybody responds well to cancer immunotherapy, but also some patients are completely cured. So, the question is, are there any predictive biomarkers that we can identify?”
says Zeynep H. Gümüş, PhD, Associate Professor in the Department of Genetics and Genomics, the Precision Immunology Institute (PRIISM) and the Tisch Cancer Institute at Mount Sinai. “In order to do that, we need to be able to understand the data.”

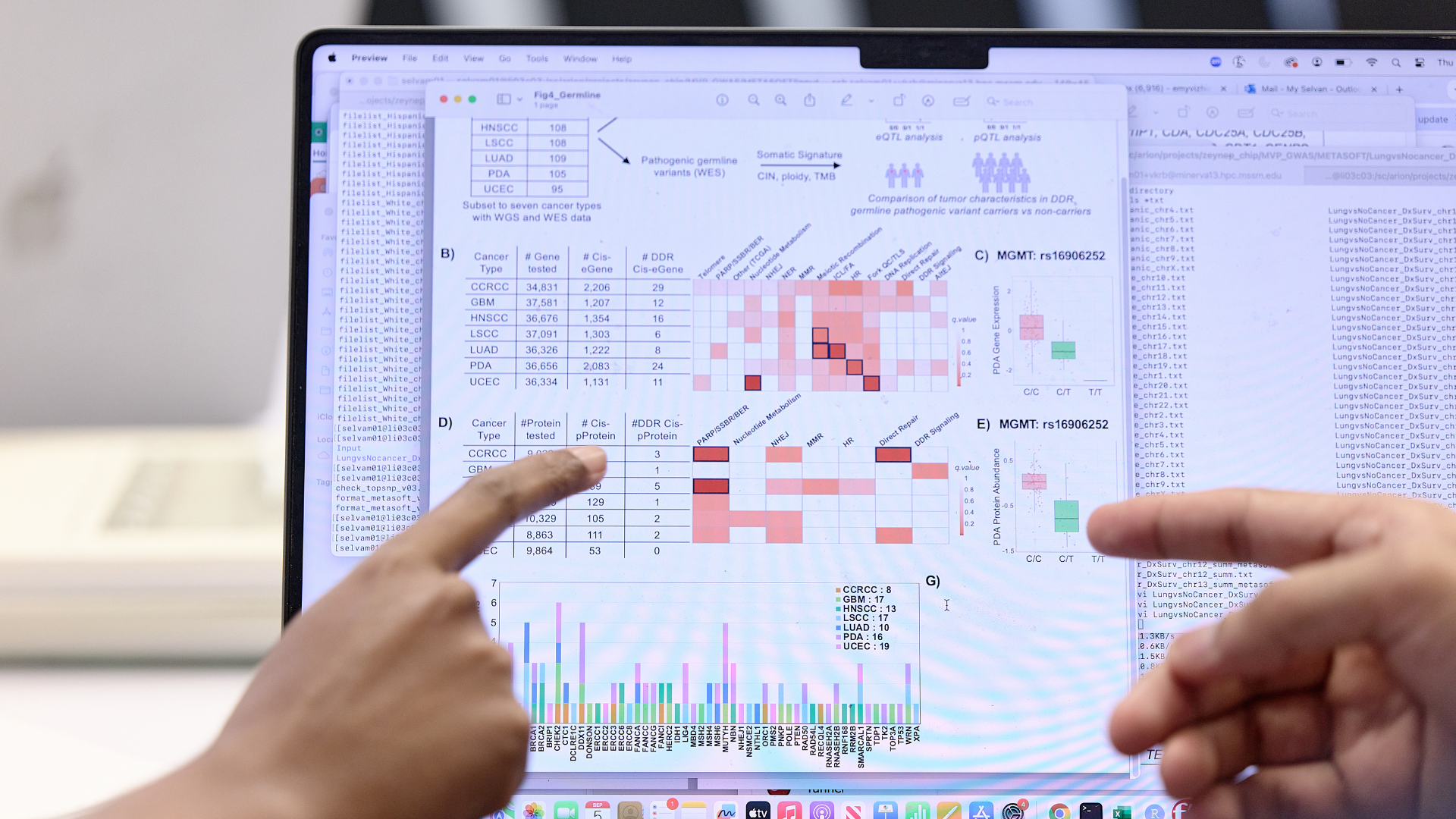
PRIMAVO is a web-based tool for the visual analysis and interactive exploration of tumor and immune profiles of cancer patients in immunotherapy clinical trials. It contains data from whole-exome sequencing, RNA-sequencing, protein abundance, antibody levels, and more measured at multiple time points, and collected as part of Cancer Moonshot Initiative of the National Cancer Institute. It lowers the barriers to handling complex data from over 30 clinical trials on cancer immunotherapy, enabling researchers to uncover biological insights and actionable discoveries.
“That’s where the Department of Genetics and Genomics shines. Because we have a large body of investigators applying different genetic or genomic approaches to different diseases, even if I’m not working in a specific field, I can still learn from my peers,” Gümüş says.
“So, there’s a vast amount of intellectual capital that we can build on.”
