Among the serious health challenges people with inflammatory bowel disease (IBD) face is increased risk of colorectal cancer, which can impose a lifetime burden of colonoscopies on younger patients. For the gastroenterologists who treat them, an added challenge is the fact that active inflammation often makes it difficult to detect dysplasia, even with modern endoscopic imaging techniques.
According to a new study by Mount Sinai and Mayo Clinic researchers, a multi-target stool-based DNA test that has become commercially popular offers promise as a complement to colonoscopic evaluations for patients with ulcerative colitis (UC) and Crohn’s disease (CD). The investigation was designed to determine if the DNA markers incorporated into the test—but without the hemoglobin content, which can lead to false positive readings—could be useful for colorectal neoplasia detection in people with IBD. The study was published in October 2023 in the Journal of Crohn’s and Colitis.
“In our proof-of-concept study, we learned that the stool-based DNA test performed well, but may need to be refined to be a highly useful diagnostic tool for the many patients who may prefer a stool-based test,” says Steven Itzkowitz, MD, lead author of the study and Professor of Medicine (Gastroenterology), Oncological Sciences, and Medical Education, at the Icahn School of Medicine at Mount Sinai. “Interestingly, the test was 100 percent effective in picking up high-grade dysplasia in our patient cohort—a terrific result because we want to catch these cases before they become cancer.”
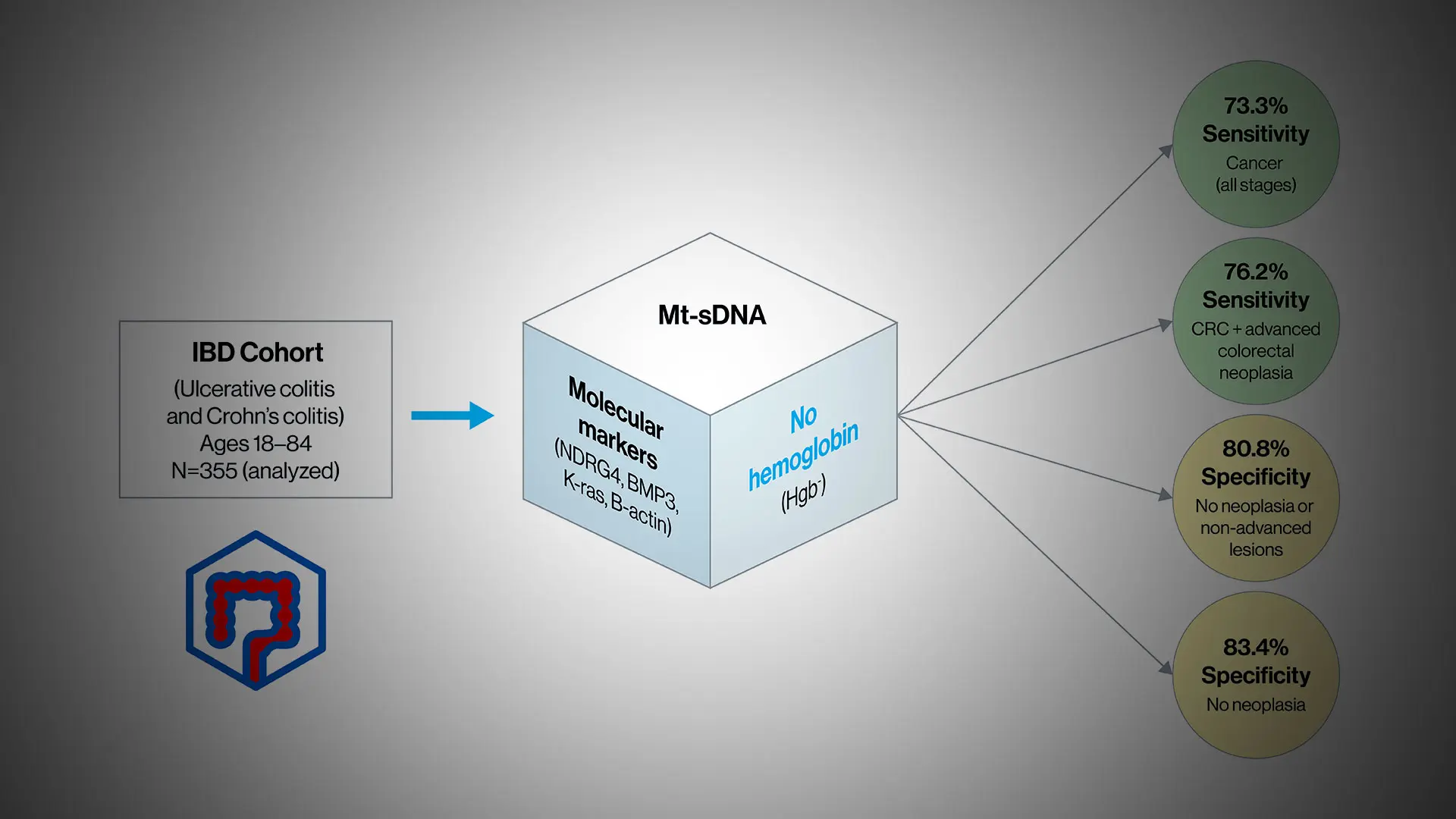
The multi-target stool-based DNA test was approved by the U.S. Food and Drug Administration in 2014 for screening average-risk patients 45 years or older for colorectal cancer, but not for use in patients with IBD under its current formulation, which incorporates detection of hemoglobin in the stool. That’s because intestinal bleeding, which can occur frequently in patients with UC and CD, could skew the hemoglobin detection part of the molecular assay, producing false positives in people who are actually free of cancer or dysplasia. For their multicenter study of 355 patients with IBD, the researchers therefore removed hemoglobin from the product’s DNA biomarker panel to develop a clinically useful IBD-specific surveillance test for colorectal neoplasia.
A stool-based test achieved 100 percent detection of lesions with high-grade dysplasia alone.
The results were encouraging. They showed that the new formulation—using the molecular markers NDRG4, BMP3, and K-ras, but without hemoglobin—had a sensitivity of 73.3 percent for colorectal cancer at all stages of the disease. When combined with screening for high-grade dysplasia, the precursor to cancer, sensitivity rose to 78.6 percent. Most exciting for the researchers, though, was the finding that the stool-based test achieved 100 percent detection in the case of lesions with high-grade dysplasia alone. Specificity for colorectal cancer was also high, at 80.8 percent, but here Dr. Itzkowitz acknowledges a caveat.
“The flip side of 81 percent specificity is that 19 percent of people will have a false positive as determined by follow-up colonoscopies showing no abnormality, compared to 13 percent false positives in the general population,” he notes. “But if we do a good-quality colonoscopy and find absolutely nothing wrong after a positive stool-based DNA test, we feel comfortable telling a patient in the general population it was a false positive and that they’re fine. For patients with IBD, however, a false positive may mean that precancerous or even cancerous lesions have been missed, so patients still need close colonoscopic surveillance.”
Dr. Itzkowitz, who is also chair of the American Cancer Society National Colorectal Cancer Roundtable, emphasizes that colonoscopy is still the gold standard for colorectal cancer testing in the adult population. Part of the reason is that colonoscopy has the ability to pick up the vast majority of polyps compared to multi-target stool-based DNA tests, which miss about half of them. Still, the latter have grown in popularity nationally because of the convenience they offer patients through ease of access, no missed time from work, noninvasiveness, and no bowel purging.
Work is ongoing in the lab to develop next-generation assays to provide even greater accuracy in screening for colorectal cancer in IBD patients, with the study’s senior author, John Kisiel, MD, a gastroenterologist at the Mayo Clinic, in the vanguard. “We’ve demonstrated that stool-based DNA testing combined with surveillance colonoscopy offers distinct advantages over the standard of care,” elaborates Dr. Itzkowitz, “and with anticipated refinements in molecular markers, we believe stool-based tests could eventually become a welcome part of clinical practice in patients with IBD.”
Featured

Steven Itzkowitz, MD
Professor of Medicine (Gastroenterology), Oncological Sciences, and Medical Education