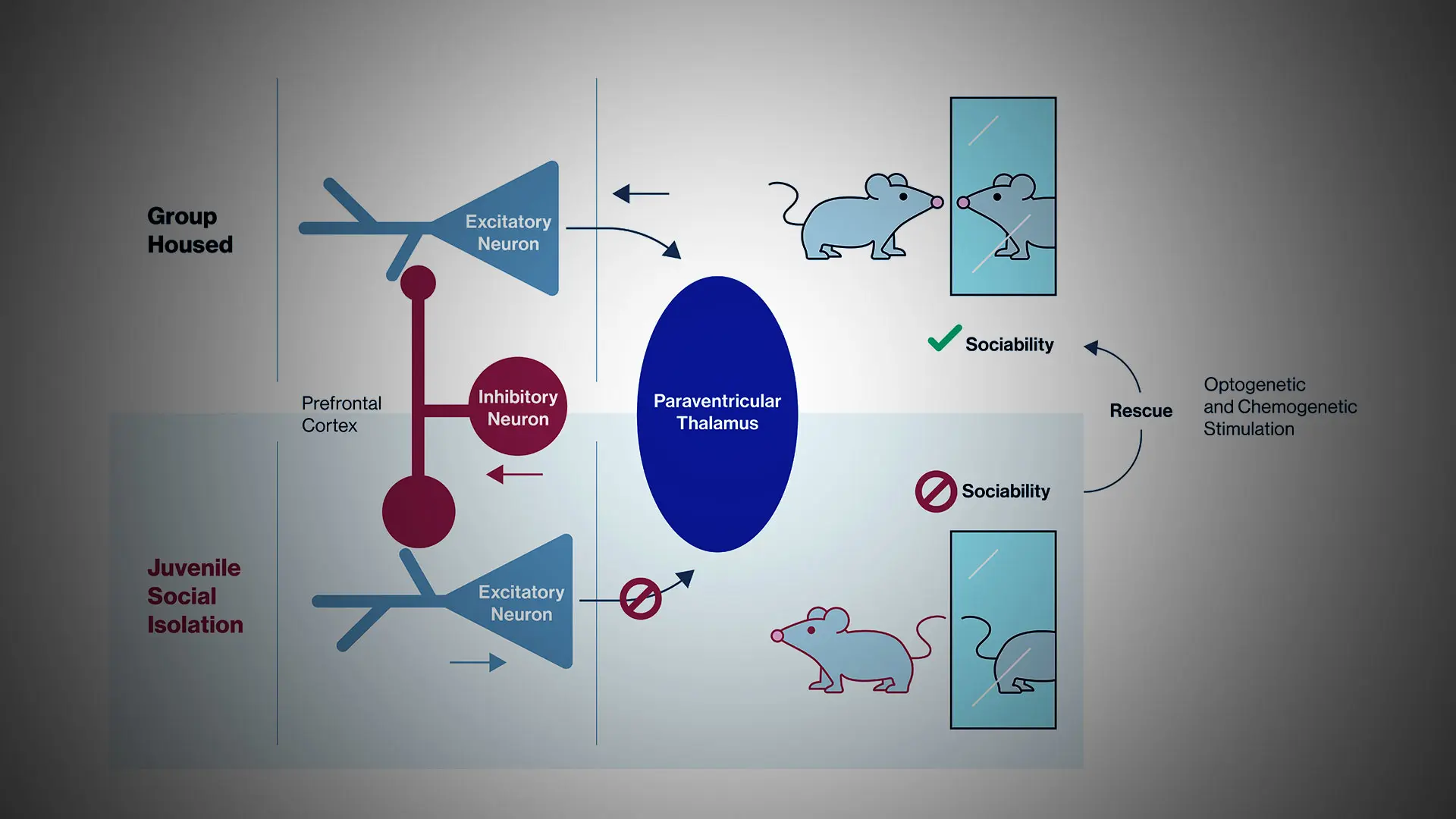
While research has shown that social isolation during childhood in particular is detrimental to adult brain function and behavior across mammalian species, the underlying neural circuit mechanisms have remained poorly understood.
We identified subpopulations of brain cells in the prefrontal cortex that are required for normal …
… sociability in adulthood and are profoundly vulnerable to juvenile social isolation in mice.
A research team at Mount Sinai led by Hirofumi Morishita, MD, PhD, Professor of Psychiatry, Ophthalmology, and Neuroscience at the Icahn School of Medicine at Mount Sinai, has identified specific subpopulations of brain cells in the prefrontal cortex, a key part of the brain that regulates social behavior, that are required for normal sociability in adulthood and are profoundly vulnerable to juvenile social isolation in mice.
Their findings shed light on a previously unrecognized role of these cells—medial prefrontal cortex pyramidal neurons that project to the paraventricular nucleus of the thalamus, the brain area that relays signals to several components of the brain’s reward circuitry (see figure below).
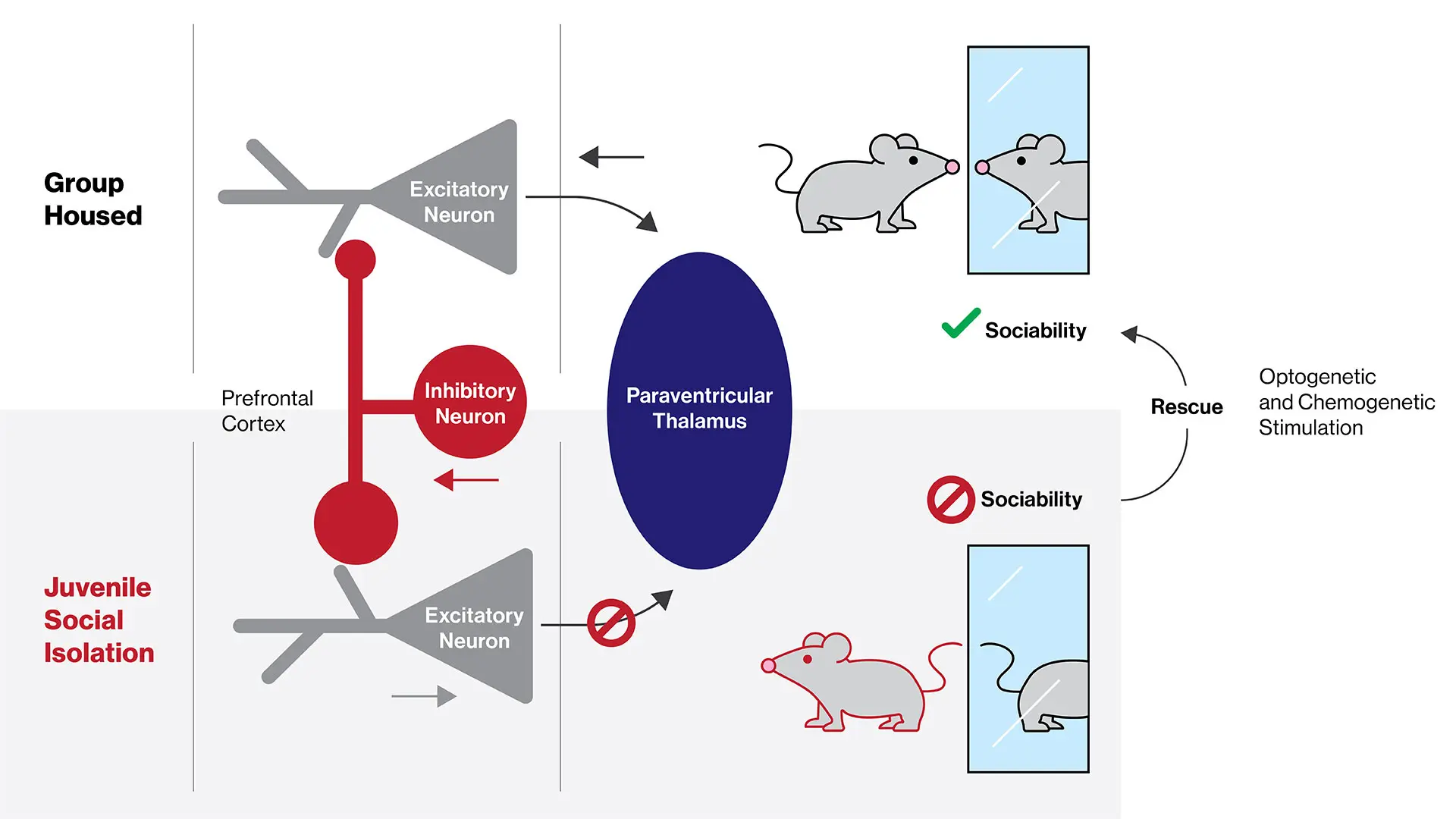
The team additionally has demonstrated that this vulnerable circuit is a promising target for treatments of deficits in social behavior by employing a technique known as optogenetics to selectively stimulate the prefrontal pyramidal neurons that project to the paraventricular thalamus. The researchers also have used chemogenetics in their studies. While optogenetics enables researchers to stimulate particular neurons in freely moving animals with pulses of light, chemogenetics allows noninvasive chemical control over the same cell populations. By employing both of these complementary techniques, the researchers were able to quickly restore social interaction in these mice upon light or chemical activation of this microcircuit.
Investigators at Mount Sinai’s Seaver Autism Center for Research and Treatment, including Hala Harony-Nicolas, PhD, Associate Professor of Psychiatry, and Neuroscience; Silvia De Rubeis, PhD, Associate Professor of Psychiatry; and Director Joseph Buxbaum, PhD, the G. Harold and Leila Y. Mathers Research Professor of Geriatrics and Adult Development, and Professor of Psychiatry, Genetics and Genomic Sciences, and Neuroscience, are currently examining whether similar behavioral and circuit differences occur in rodents carrying mutations that are linked to autism spectrum disorder in humans. Affected individuals are known to display deficits in social communication and interactions.
In the Department of Neuroscience, two new faculty recruits—Herbert Zheng Wu, PhD, who joined Mount Sinai from Columbia University, and Xiaoting Wu, PhD, who joined from Stanford University, each an Assistant Professor of Neuroscience—recently initiated new research programs to identify neural circuits that are essential for social decision-making or social memory using mouse models. Dr. Herbert Zheng Wu was selected to receive a 2023 Sloan Research Fellowship from the Alfred P. Sloan Foundation, which honors outstanding scientists who are making significant contributions early in their careers.
The circuits identified in this study have direct clinical applications.
Given that social behavior deficits are a common facet of many neurodevelopmental and psychiatric disorders, such as autism, schizophrenia, depression, and anxiety, identification of distinct subpopulations of prefrontal cortical neurons and their specific projections will point toward therapeutic targets for the improvement of social behavior deficits shared across a range of psychiatric conditions. The circuits identified in these investigators’ work have direct clinical applications, since they could potentially be modulated using established therapeutic techniques such as transcranial magnetic stimulation or transcranial direct current stimulation.
Featured

Hirofumi Morishita, MD, PhD
Professor of Psychiatry, Ophthalmology, and Neuroscience, Icahn School of Medicine at Mount Sinai

Hala Harony-Nicolas, PhD
Associate Professor of Psychiatry, and Neuroscience, Icahn School of Medicine at Mount Sinai

Silvia De Rubeis, PhD
Associate Professor of Psychiatry, Icahn School of Medicine at Mount Sinai

Joseph Buxbaum, PhD
Director, Seaver Autism Center for Research and Treatment; G. Harold and Leila Y. Mathers Research Professor of Geriatrics and Adult Development; and Professor of Psychiatry, Genetics and Genomic Sciences, and Neuroscience

Herbert Zheng Wu, PhD
Assistant Professor of Neuroscience, Icahn School of Medicine at Mount Sinai

Xiaoting Wu, PhD
Assistant Professor of Neuroscience, Icahn School of Medicine at Mount Sinai