Unfortunately, however, direct causal relationships between chromatin regulatory phenomena and neuropathology have often been difficult to ascertain owing, in part, to the diverse heterogeneity of cell populations within the brain, as well as the non-specific and genome-wide effects induced by classical approaches used to manipulate the epigenome.
The emergence of advanced chemical and molecular technologies…
…now allows us to directly modify the epigenomic landscape.
Progress toward the field’s ultimate goal of being able to “drug” the neural epigenome has remained largely unrealized, but with the emergence of advanced chemical and molecular technologies that now allows investigators to directly modify the epigenomic landscape, often in a cell-type and locus-specific manner, this reality is beginning to change.
For example, epigenetic editing technologies have improved dramatically in the last several years through the application of CRISPR- (clustered regularly interspaced short palindromic repeats) based tools. Through implementation of CRISPR/dCas9 systems fused to DNA sequence recognition domains, it is now possible to deliver conjugated domains of enzymatic activators or repressors of gene expression to targeted loci to assess their impact on gene regulation in the brain through the artificial establishment of endogenous chromatin modifications.
Such methodologies, pioneered in the brain by Eric J. Nestler, MD, PhD, and his colleagues, can be further enhanced by the latest protein engineering strategies aimed at using protein structural information (for example, chromatin effector:chromatin modification interactions) to increase the specificity or magnitude of genomic manipulations. Ultimately, such tools could be delivered to humans as a way to correct gene expression abnormalities.
In addition to these approaches, which rely on both artificial and endogenous cellular components to manipulate chromatin modifications in vivo, our ability to de novo generate such modifications in a cell type-specific manner—in a single or combinational fashion— without needing to rely on unpredictable endogenous components, is also emerging in the form of a new methodology known as “protein trans-splicing.”
Protein trans-splicing employs ultra-fast-acting inteins, derived from a class of naturally occurring polypeptides, to incorporate non-native chromatin posttranslational modifications (PTMs) (for example, histone PTMs) into chromatin in living cells.
The name intein is derived from “protein introns,” and these novel molecules cleave themselves from host proteins (exteins) to generate novel-spliced protein products. With this new method, it is now possible to synthesize chromatin carrying an array of specific PTMs in vivo.
Using multiple technologies and methodologies…
…we can target small molecules to specific genomic locations.
These protein trans-splicing technologies can also be coupled to CRISPR-based methodologies to target small molecules to specific genomic locations, thereby providing a direct opportunity to begin drugging the neural epigenome in live animals (and perhaps, in the future, even in humans). Approaches like these carry with them the exciting possibility of being able to directly assess the contributions of specific chromatin marks in the brain to neuronal patterns of transcription, development, and behavior, both in normal and pathophysiological states.
Significant role for the Center for Neural Epigenome Engineering
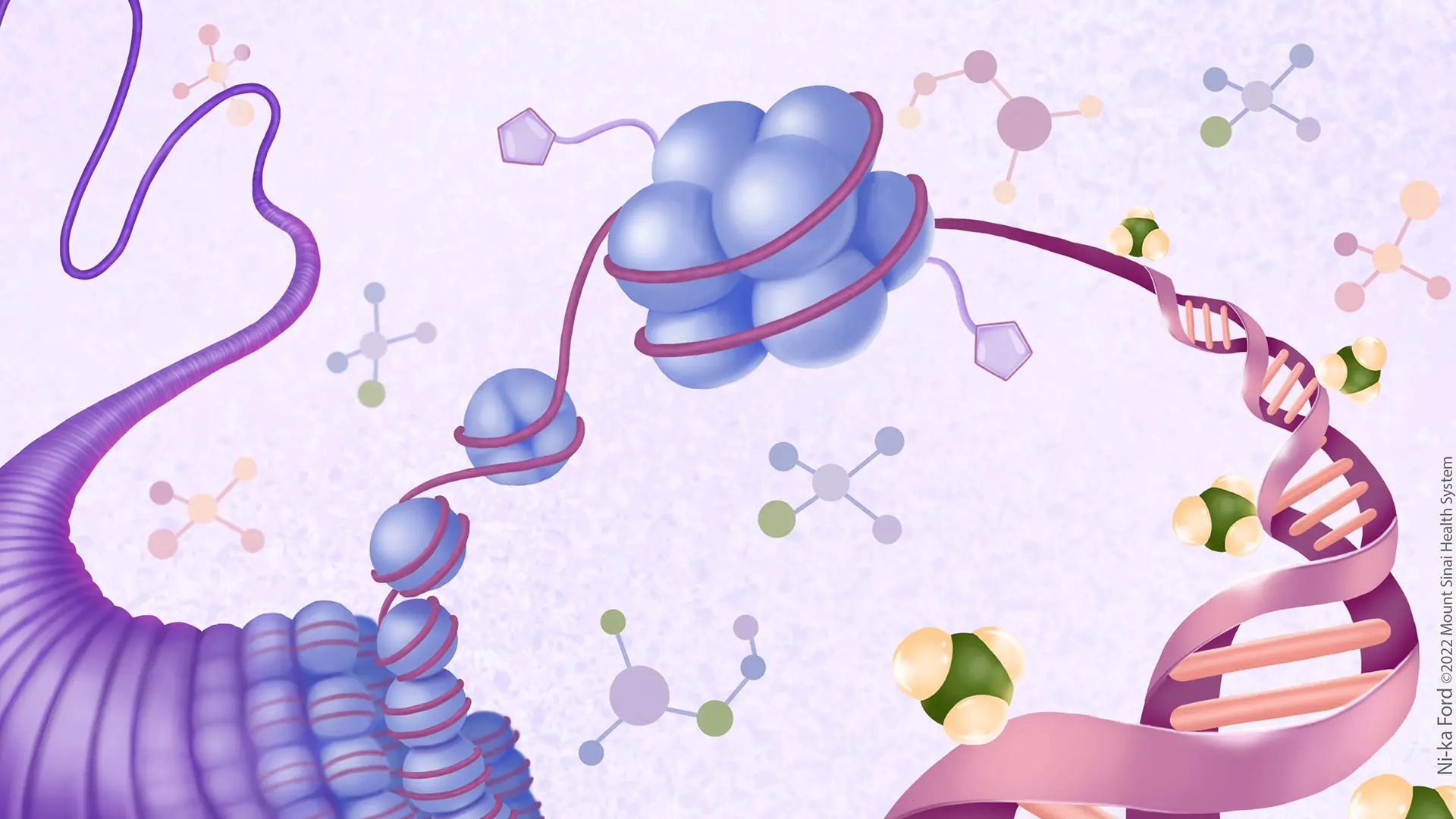
Above: Neuroepigenetic processes play critical roles in neural development and plasticity-related modifications of the adult brain. Aberrations in these processes can produce devastating neurological and psychiatric disorders. Integrating new technology and methodologies in chemical biology, proteomics, protein biochemistry/engineering, and structural biology can accelerate the discovery of mechanisms responsible for neurodevelopmental and neuropsychiatric illnesses and the development of targeted neurotherapeutics. Credit: Center for Neural Epigenome Engineering, by Ni-ka Ford
This line of research is being driven by Ian S. Maze, PhD, who was elected a Howard Hughes Medical Institute Investigator in fall 2021. As Director of the newly inaugurated Center for Neural Epigenome Engineering, Dr. Maze and his team are dramatically expanding capabilities in the most exciting areas of chromatin biochemistry, chemical biology, protein engineering, and single-cell omics (including spatial transcriptomics) as they relate to studies of the CNS as well as the peripheral nervous system.
This novel Center will catalyze the integration of the most technologically innovative and sophisticated new methodologies to drug discovery platforms. This approach promises to accelerate the pace of discovery of mechanisms responsible for a wide range of neurological and psychiatric illnesses and to facilitate the development of highly novel classes of targeted neurotherapeutics.