The impact of brain cancer is felt by everyone and, in comparison to many other forms of cancer, little progress has been made in terms of curative treatments for both adult and pediatric patients.
The most common primary brain cancer that originates in adults, known as glioblastoma (GBM), is all too familiar in its devastating effect with patients. In children, two forms of brain cancer known as diffuse intrinsic pontine glioma (DIPG) and medulloblastoma are equally devastating. The vast majority of patients have recurrence of their tumors despite surgery, radiation, and chemotherapy.
A major challenge is that these tumors have infiltrative or invasive growth patterns into the surrounding brain that are difficult to identify and treat, resulting in tumor recurrence. In addition, the blood-brain barrier (BBB) prevents many therapies delivered systemically from getting into the brain to attack the tumors, and the immune system is often suppressed both within and around brain tumors, which reduces the body’s natural mechanisms of fighting the illness.
The Brain Cancer Working Group is a new collaboration at Mount Sinai.
Investigators at Mount Sinai are working to make a difference with new approaches to treat and prevent regrowth of these very difficult cancers. Recruitment of new faculty, and full integration of efforts between basic researchers and clinician-scientists at Mount Sinai, have resulted in the development of a leading multidisciplinary team, major new funding from the National Institutes of Health (NIH), and promising efforts to better understand and ultimately conquer adult and pediatric brain cancer.
Here we provide an overview of this exciting research and clinical work that is being carried out under the auspices of a newly formed Brain Cancer Working Group––a collaborative effort between The Friedman Brain Institute and The Tisch Cancer Institute, involving numerous departments, including Neurosurgery, Neurology, Neuroscience, Oncological Sciences, Pediatrics, and Pathology, as well as The Black Family Stem Cell Institute and the Precision Immunology Institute at Mount Sinai.
Targeting Brain Cancer Cell Invasion
Nadejda (Nadia) Tsankova, MD, PhD, newly named Chief of Neuropathology, discovered that a transcription factor involved with the Hippo signaling pathway, TEAD1, is a critical regulator of migration or invasion in GBM. The Tsankova laboratory recently demonstrated the ability of a U.S. Food and Drug Administration (FDA)-approved TEAD inhibitor, verteporfin—which is marketed to reduce excess blood vessels in the treatment of macular degeneration—to reduce tumor invasiveness, exert a therapeutic-like effect, and increase survival across GBM models in rodents. Dr. Tsankova is currently collaborating with Constantinos G. Hadjipanayis, MD, PhD, Director of Neurosurgical Oncology, to initiate a pilot clinical trial studying verteporfin as an adjuvant chemotherapeutic agent in patients with recurrent GBM.
Roland Friedel, PhD, Associate Professor of Neuroscience, and Neurosurgery, has also identified novel molecules that regulate the invasive spreading behavior of brain cancer cells. One molecule is Plexin-B2, which is an axon guidance receptor that is highly expressed in GBM tumors (Fig. 1), and facilitates the invasive growth of GBM cells into normal brain tissue. Recent discoveries on biomechanical roles of plexin family proteins have led the Friedel laboratory to investigate how plexins provide brain cancer cells with the unique ability to penetrate through restricted spaces in the brain, enabling tumor spread. The laboratory is now focusing on how to target Plexin-B2 as a potential treatment of GBM.
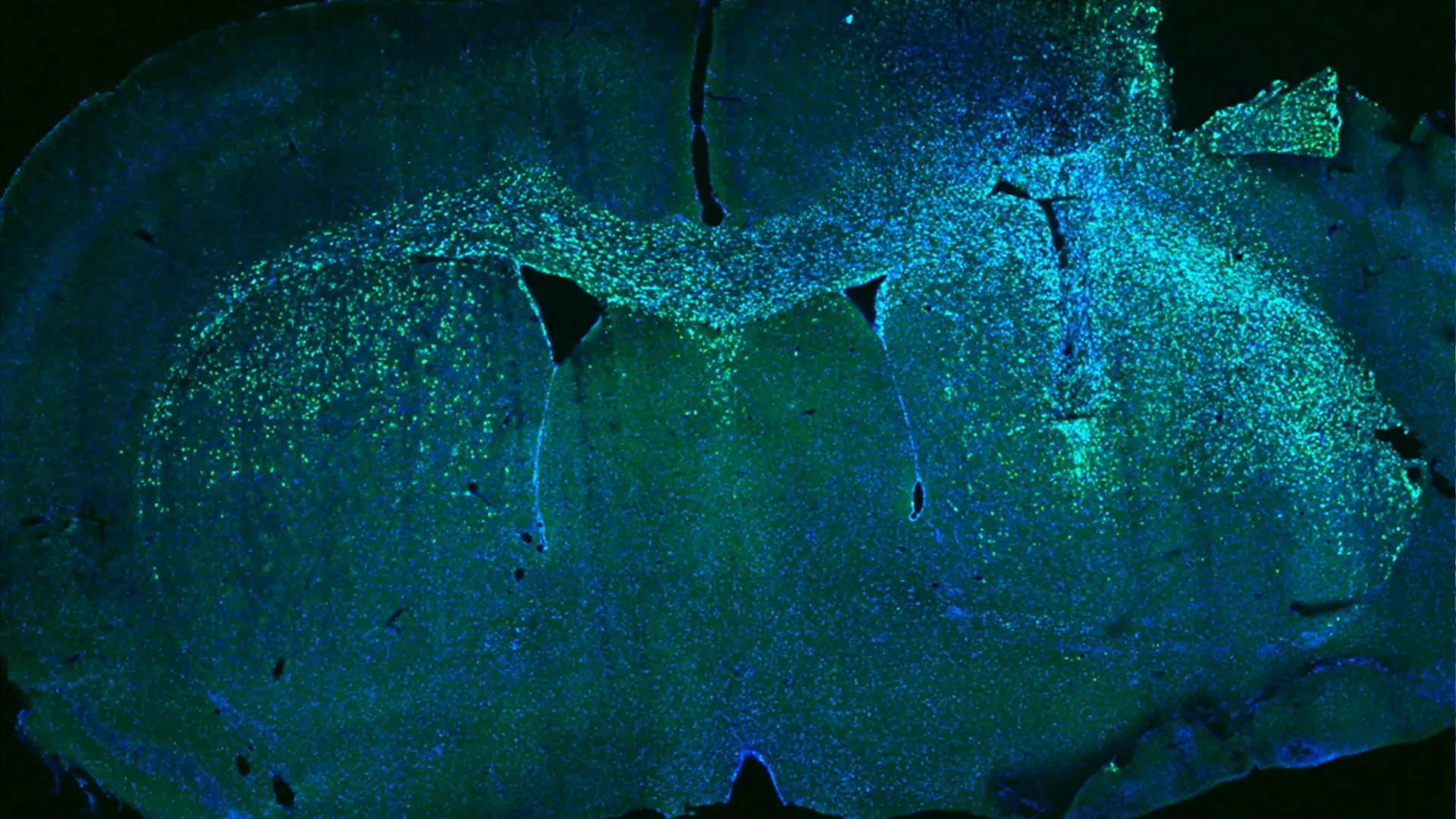
Fig. 1. Human glioblastoma cells were transplanted into the right side of a mouse brain. The glioblastoma cells (green cells in a blue-stained brain section) recapitulate the invasive growth that is seen in patients and have crossed through the midline to invade the left hemisphere.
Understanding the Tumor Microenvironment and Immunosuppression
Hongyan Zou, MD, PhD, Professor of Neurosurgery, and Neuroscience, and an active neurosurgeon, studies the immune landscape and how dormant or quiescent tumor cells constitute potential sources for tumor recurrence. Her laboratory has teamed up with bioengineers to develop novel biomimetic 3D glioma vascular models to dissect governing factors of glioma quiescence.
Dr. Zou hypothesizes that the immune landscape and the surrounding extracellular matrix can determine tumor quiescence versus recurrence. Her laboratory is also studying how hypoxia in the tumor microenvironment is a critical link for the reciprocal communication between GBM and immune cells that enhances malignant potency and immunosuppression within the tumor (Fig. 2). Myeloid cells, known as tumor-associated macrophages (TAMs), can be identified in GBM tumors by the CD68 transmembrane glycoprotein marker. She is testing novel strategies to target the hypoxic microenvironment to sensitize GBM cells to conventional chemotherapies.
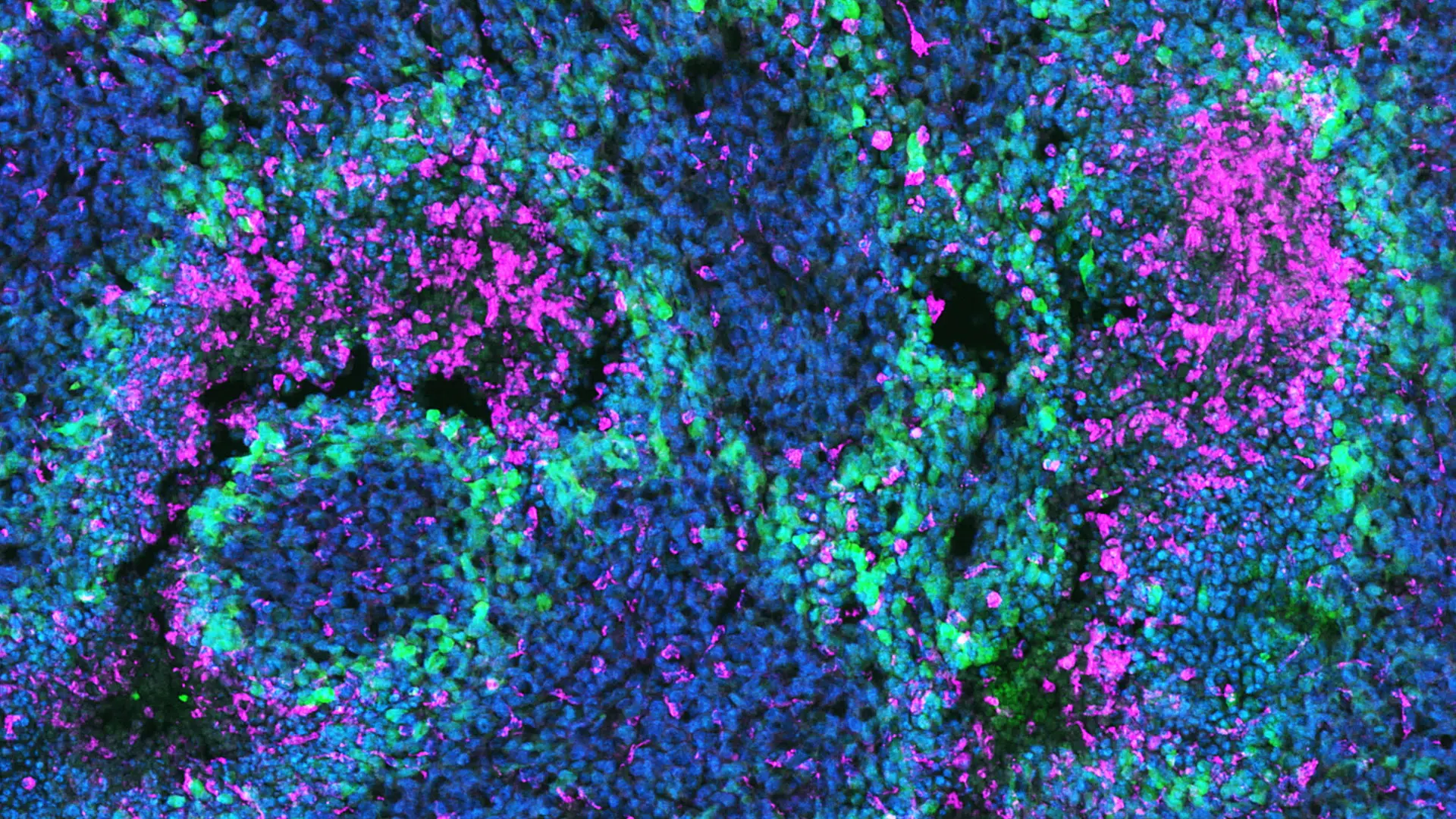
Fig. 2. Immunofluorescent image of a human glioblastoma (GBM) showing sequestration of CD68+ tumor associated macrophages (red) by hypoxic GBM cells in pseudopallisading patterns (green).
Dolores Hambardzumyan, PhD, Associate Professor of Neurosurgery, and Oncological Sciences, who joined Mount Sinai recently from Emory University, has made significant advances in understanding how immunosuppression can occur with brain cancer. Her laboratory is defining the origin and role of brain-resident microglia and infiltrating myeloid cells in GBM tumors (Fig. 3).
Both types of myeloid cells are components of the innate immune system and are thought to represent important potential targets in the treatment of GBM. Dr. Hambardzumyan has also developed important genetically-engineered mouse models of GBM and medulloblastomas. These models are based on RCAS/TVA, a cell-type-specific viral gene transfer system. The modified mice closely resemble their human counterparts in molecular profiling, imaging properties, and gross pathology of the tumors. Investigators collaborate from different
institutions with Dr. Hambardzumyan and utilize the mouse lines to define driver mutations in adult and pediatric brain cancer with the goal of understanding how tumors form and grow in the central nervous system.
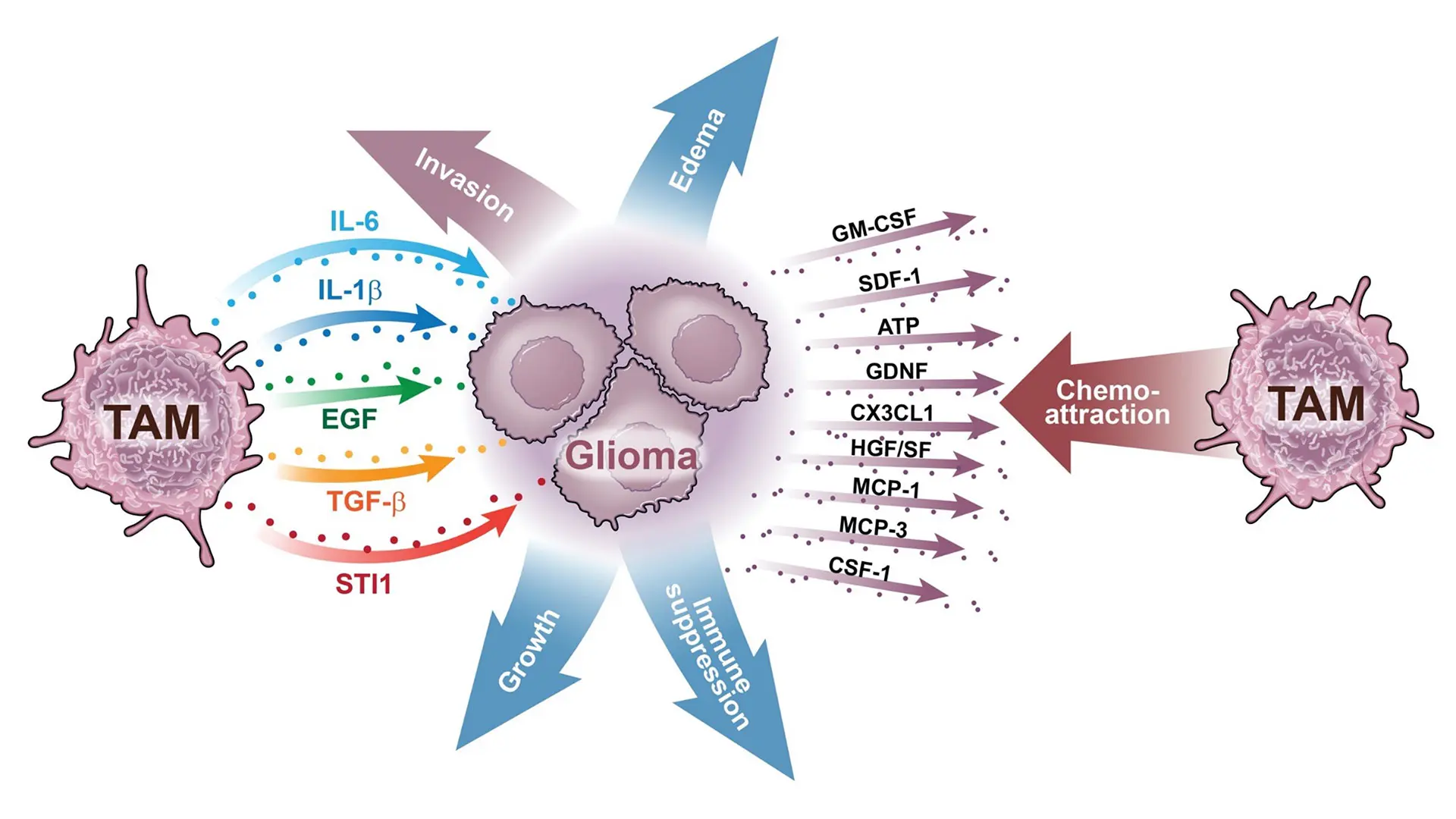
Fig. 3. Tumor-associated macrophages (TAM) and their association with glioma cells. Shown here are numerous TAM-derived factors that influence glioma cells and, conversely, numerous glioma-derived factors that influence TAM function.
Translational Studies
Dr. Hadjipanayis and his laboratory focus on the development of novel therapeutic approaches for GBM tumors. One such approach involves the use of magnetic iron-oxide nanoparticles and the application of an alternating magnetic field to generate local hyperthermia during neurosurgery for treatment of brain cancer. This approach is known as magnetic hyperthermia therapy (MHT) and may permit better treatment of GBM and enhance the effects of radiation and chemotherapy. Dr. Hadjipanayis and his collaborators at Johns Hopkins University and Pennsylvania State University are initiating a canine glioma clinical trial to study how to apply this treatment to patients.
Raymund Yong, MD, Assistant Professor of Neurosurgery, and Oncological Sciences, is developing innovative strategies that combine epigenetic and traditional chemotherapeutic agents for better treatment of GBM tumors. Dr. Yong is currently focused on improving GBM responses to temozolomide (which adds alkyl/methyl groups to DNA), using the hypomethylating agent decitabine, which acts by potentiating DNA mismatch repair, among other mechanisms. Drs. Yong and Hadjipanayis are collaborating to initiate a first-of-its-kind clinical trial in the United States for GBM patients that will study the use of intraoperative photodynamic therapy (PDT) after tumor resection (Fig. 4). This will entail the use of the photosensitive agent 5-ALA (5-aminolevulinic acid) that is already FDA-approved for fluorescence-guided surgery (FGS) in glioma patients.

Fig. 4 5-ALA intraoperative photodynamic therapy for glioblastoma
patients
Immunotherapy
A promising Phase 1 vaccine immunotherapy study has been developed at Mount Sinai for treatment of new GBM patients. A fully personalized therapeutic vaccine is developed from mutation-derived tumor antigens (MTA) found in a patient’s GBM tumor that arise during tumor formation. These somatic mutations are characterized by genome sequencing, and the resulting data are analyzed to identify the MTAs. The vaccine is administered to patients after completion of standard of care treatments (for example, surgery, radiotherapy, and concurrent temozolomide chemotherapy) and is given with adjuvant temozolomide chemotherapy and alternating electric tumor treatment fields. Nina Bhardwaj, MD, PhD, Director of Immunotherapy and Medical Director of the Vaccine and Cell Therapy Laboratory in The Tisch Cancer Institute, has been instrumental in the production of these personalized vaccines for GBM patients.
Pediatric Brain Cancer and the BBB
Oren J. Becher, MD, the Steven Ravitch Chair in Pediatric Hematology and Division Chief of Pediatric Hematology-Oncology, who recently joined Mount Sinai from the Feinberg School of Medicine at Northwestern University, explores the function of genetic mutations and epigenetic modifiers in pediatric brain cancers with a focus on DIPG tumors. Dr. Becher’s laboratory co-discovered the presence of activating mutations in ACVR1, which encodes Activin A receptor type 1 and is found in 25 percent of children with DIPG. His group has developed mutant mice harboring ACVR1 mutations, as well as mutations in the epigenetic mark H3K27me3 (histone H3 Lys27 trimethylation), abnormalities that are a hallmark of DIPG. Dr. Becher has successfully used these models to study the tumor microenvironment and evaluate novel therapeutics to help prioritize clinical trials for children with DIPG. He and his team at Mount Sinai seek to initiate new clinical trials for children with brain cancers.
Praveen B. Raju, MD, PhD, Associate Professor of Neurology, and Pediatrics, concentrates on the other major type of pediatric brain cancer known as medulloblastoma. His research group uses sophisticated mouse genetic approaches to create more physiologically relevant animal models of the disease to study medulloblastoma cells and their microenvironment at single-cell resolution. This approach allows assessment of medulloblastoma biology both during tumor evolution to better understand cellular composition, as well as following respective therapies to identify molecular mechanisms of tumor recurrence and relapse. Recent work in his group utilizes a novel nanoparticle-based approach to enhance drug delivery past the BBB to improve efficacy and minimize systemic toxicities that have been major limiting factors for the effective treatment of medulloblastoma as well as other pediatric and adult brain tumors (Fig. 5).
Dr. Constantinos Hadjipanayis is a consultant for the privately held company NX
Development Corporation, and receives royalty payments for the sale of Gleolan
(5-ALA, aminolevulinic acid hydrochloride). Gleolan is an optical imaging agent
approved for the visualization of malignant tissue during glioma surgery. NX
Development Corporation markets Gleolan. Dr. Hadjipanayis also receives
financial compensation as a consultant for Hemerion, the Stryker Corporation,
and Synaptive Medical (device companies that manufacture medical technologies used for the treatment of brain cancer).
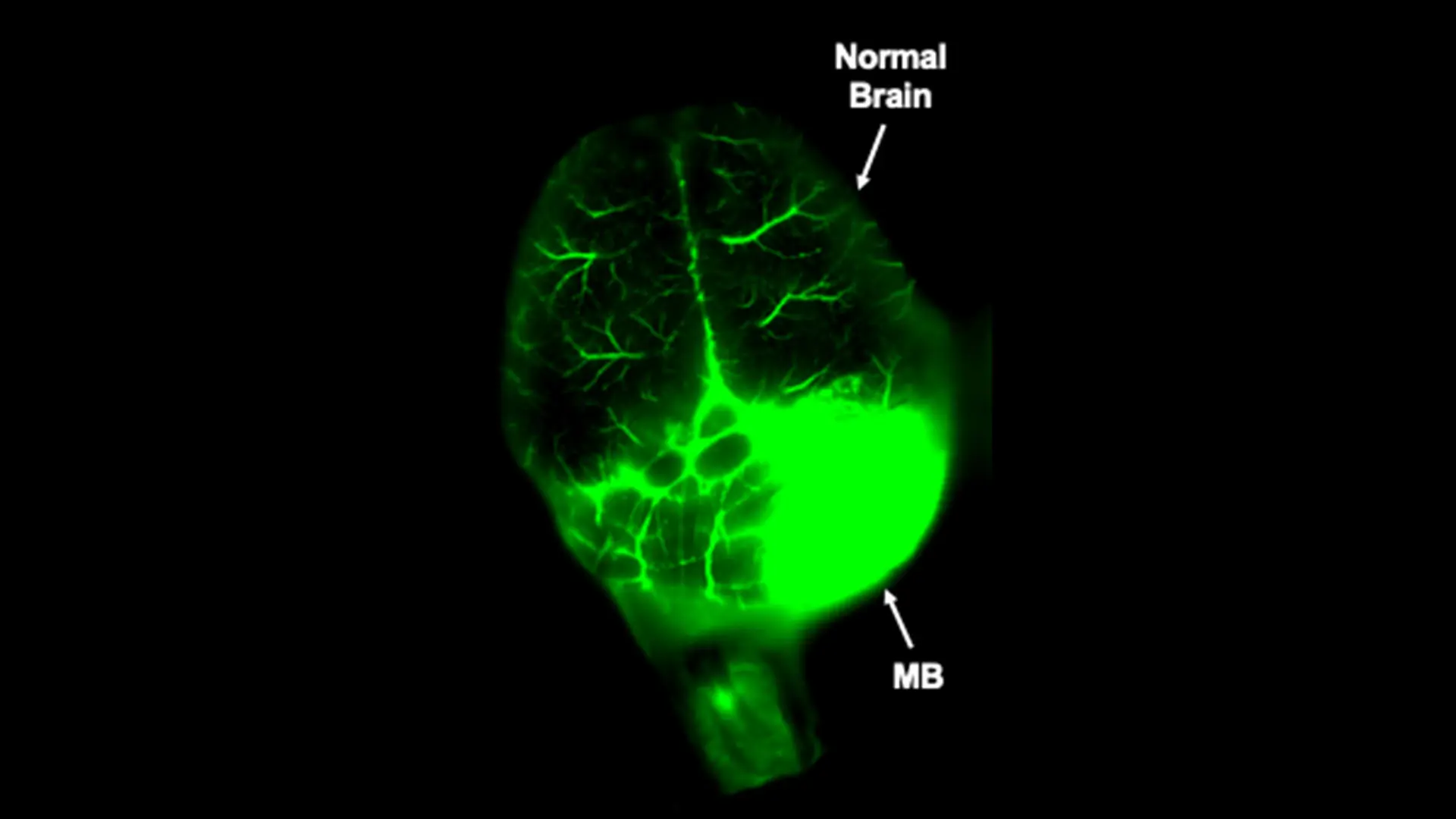
Fig. 5. Nano-targeted drug delivery (green) specifically to a medulloblastoma (MB) and not adjacent normal brain in a preclinical genetic brain tumor model with an intact blood-brain barrier.
