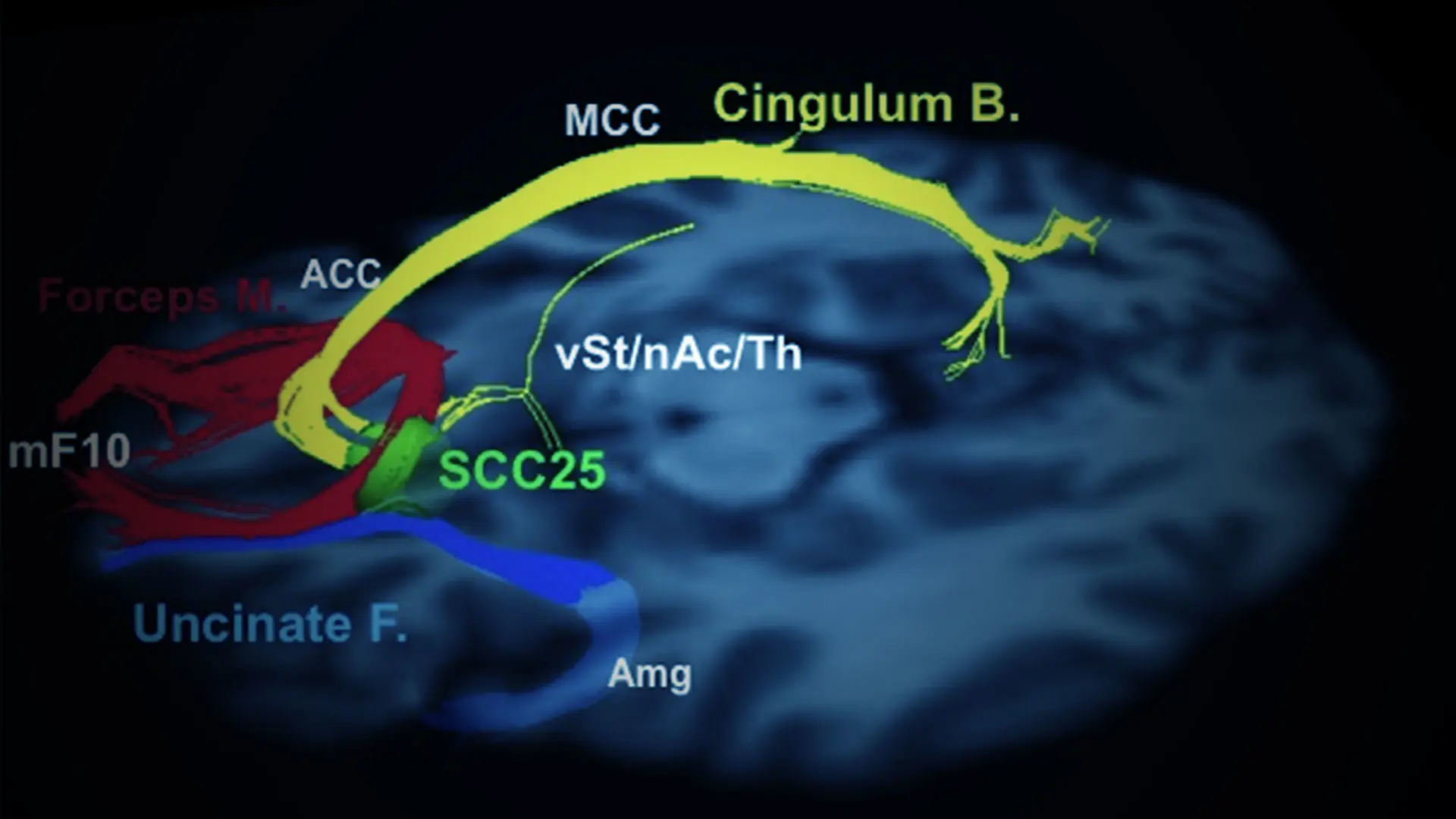
The U.S. Food and Drug Administration (FDA) granted Abbott Breakthrough Device Designation to explore use of DBS for TRD under its Breakthrough Devices Program, which expedites the review of innovative technologies that can improve the lives of people with life-threatening or irreversibly debilitating diseases or conditions.
"As we have learned more about the intricacies of the brain, it is now clear that 'psychiatric diseases' such as major depressive disorder are similar to other neurological conditions—we can see identifiable structural and functional changes in the brain," says Brian H. Kopell, MD, lead neurosurgery investigator, who is Director of the Center for Neuromodulation and Co-Director of the Bonnie and Tom Strauss Center for Movement Disorders at the Mount Sinai Health System, and Professor of Neurosurgery, Neurology, Psychiatry, and Neuroscience at the Icahn School of Medicine at Mount Sinai.
"So, it is not surprising that deep brain stimulation research has demonstrated promise for people with treatment-resistant depression, as it has for patients with medically complicated Parkinson's disease over the past two decades,” says Dr. Kopell. “We are eager for Abbott's TRANSCEND trial to gather further evidence about the impact neurostimulation could have for people who need different treatment options than are currently available."
This work has been pioneered by Helen S. Mayberg, MD, the Founding Director of the Nash Family Center for Advanced Circuit Therapeutics (C-ACT) at Mount Sinai, who is renowned for her study of brain circuits in depression and deep brain stimulation research. Dr. Mayberg is Professor of Artificial Intelligence and Human Health, Neurosurgery, Neurology, Neuroscience, and Psychiatry at Icahn Mount Sinai.
Mount Sinai is one of 25 clinical trial sites and is expected to recruit 5 to 10 TRD patients to participate in this three-year clinical trial of subcallosal cingulate cortex DBS for depression. Participants will be randomly assigned to either the treatment arm or control arm of the trial. Once a participant has received their Abbott DBS device, those in the treatment arm will have the system turned on, while those in the control arm will not. Once a participant has completed 12 months in the study, all participants will have their DBS system turned on and followed for an additional two years.
The Mount Sinai team is headed by psychiatrist Martijn Figee, MD, PhD, Associate Professor of Psychiatry, Neurosurgery, Neurology, and Neuroscience, in collaboration with other C-ACT faculty, including Dr. Kopell and Ki Sueng Choi, PhD, Associate Professor of Diagnostic, Molecular and Interventional Radiology.
Read the full announcement from Abbott here.
Featured

Brian H. Kopell, MD
Director of the Center for Neuromodulation, Co-Director of the Bonnie and Tom Strauss Center for Movement Disorders, and Professor of Neurosurgery, Neurology, Psychiatry, and Neuroscience

Helen S. Mayberg, MD
Founding Director, Nash Family Center for Advanced Circuit Therapeutics at Mount Sinai, and Professor of Artificial Intelligence and Human Health, Neurosurgery, Neurology, Neuroscience, and Psychiatry

Martijn Figee, MD, PhD
Associate Professor of Psychiatry, Neurosurgery, Neurology, and Neuroscience, and Director, Mount Sinai Interventional Psychiatry Program

Ki Sueng Choi, PhD
Associate Professor of Diagnostic, Molecular and Interventional Radiology