Highlighting the contributions made to the field by Priti Balchandani, PhD; Ignacio Saez, PhD; and Alexander Charney, MD, PhD
Brain imaging
This research area at The Friedman Brain Institute is overseen by the Advanced Neuroimaging Research Program (ANRP), which focuses on improving the performance of all neuroimaging modalities.
These include functional magnetic resonance imaging (fMRI) to study brain function, diffusion MRI to study brain networks and white matter tracts, and MR spectroscopic imaging for the study of brain metabolism. These methods are translated to diagnose and treat a wide range of central nervous system (CNS) conditions, including epilepsy, brain tumors, psychiatric illnesses, multiple sclerosis, and spinal cord injury, among many others.
Mount Sinai is also one of the few institutions that has invested in a 7 Tesla (7T) human MRI scanner, which produces high-resolution images to visualize and measure previously undetectable changes in the brain.
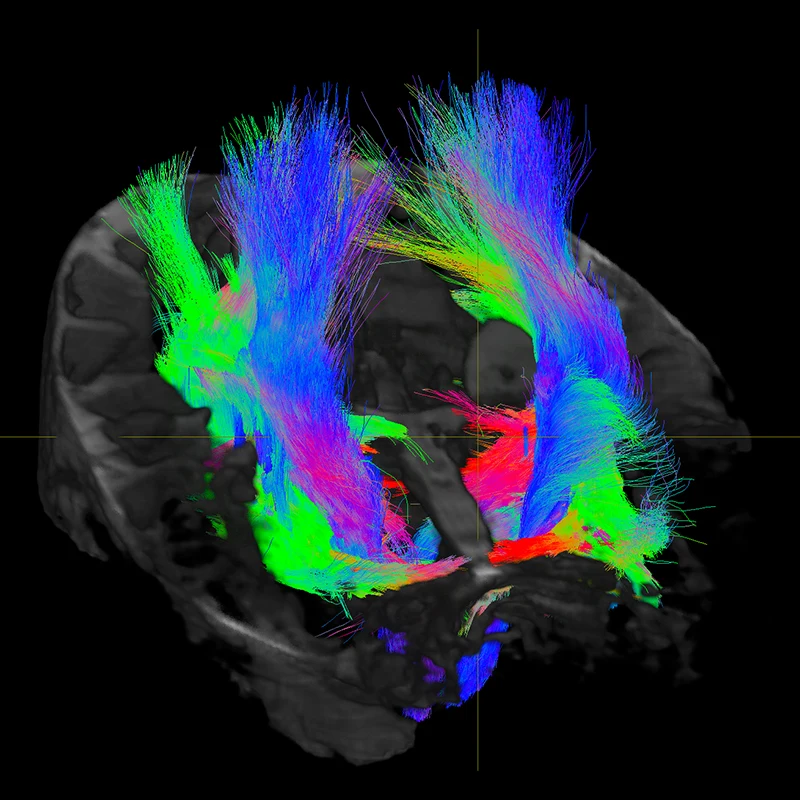
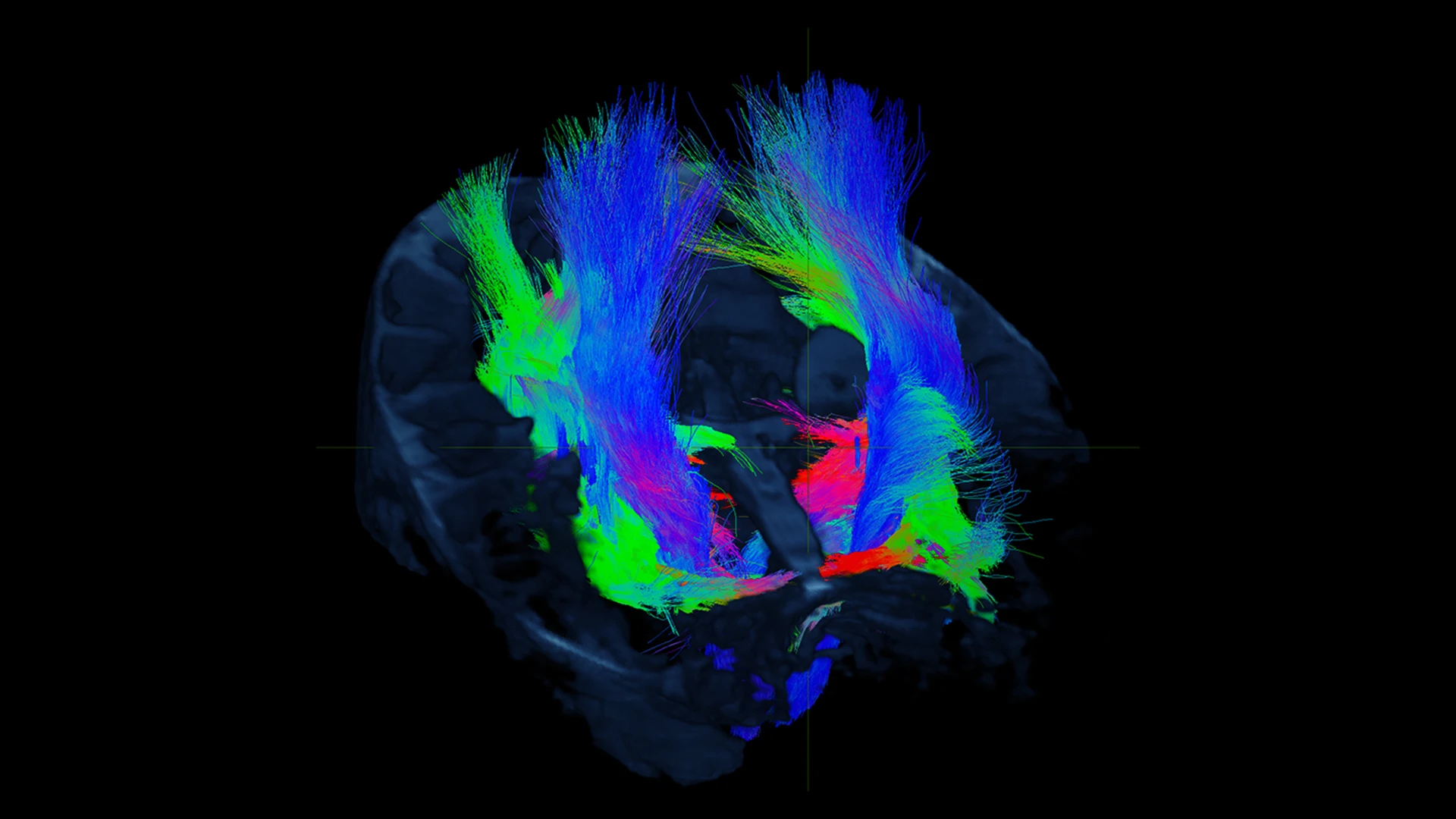
Figure 1. Diffusion MRI at 7T allows for the study of hippocampal subfield specific tracts in a living human subject.
This advanced imaging technology noninvasively captures more subtle abnormalities, enabling earlier detection of diseases that are ordinarily not uncovered through routine imaging, and earlier initiation of treatment. Using this technology also allows us to perform deeper analysis of tiny structures in the brain that are involved in multiple disease etiologies, for example, by imaging discrete subfields of the hippocampus. Figure 1 shows white matter tracts connecting to several hippocampal subfields, which are being studied in the context of Alzheimer’s disease pathology.
In Vivo neurophysiology
Another crucial window into the living human brain is the use of in vivo neurophysiology to study cognition and emotions.
Patients with intractable epilepsy have electrodes placed deep into their brains to identify the location of a seizure focus as part of evaluating whether a patient would benefit from surgical resection of that focus. Substantial evidence shows that patients with severe epilepsy do much better after surgical removal of a seizure focus compared to relying on medication treatment.
As part of this presurgical evaluation, electrodes are placed in broad regions of the temporal lobe and regions of the prefrontal cortex (for example, orbitofrontal cortex) and recordings are made over many days to several weeks (see Figure 2). Additionally, one brain area is stimulated to assess the impact on activity in other areas. This routine clinical diagnostic framework provides a unique platform to study the activity of broad regions of the temporal and frontal lobes while patients volunteer to perform a variety of cognitive and emotional tests.
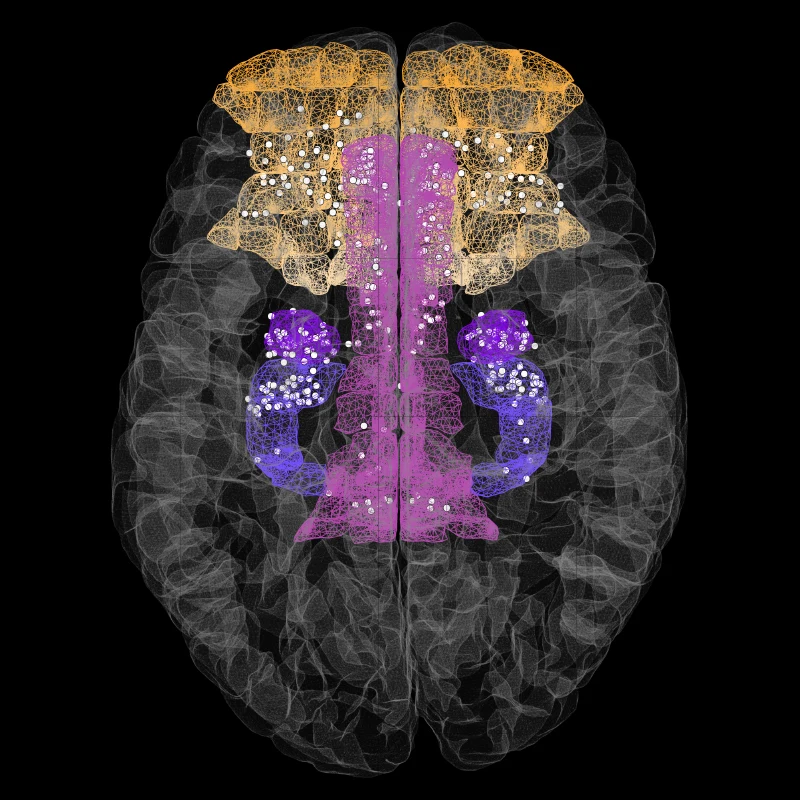
Figure 2. Composite image showing the location of invasive stereotactic electrodes (white dots) implanted deep in the brain of several patients with intractable epilepsy.
In parallel, analyzing how numerous brain areas respond when one region is stimulated offers an invaluable direct assessment of human brain connectivity that complements such studies with brain imaging. Deep brain recordings are also available from patients undergoing surgery for deep brain stimulation, now a standard treatment for Parkinson’s disease and other movement disorders, and increasingly being investigated for treatment of severe depression, obsessive-compulsive disorder, and other psychiatric syndromes.
These experiments are revealing sub-second patterns of widespread brain activity that underlie human cognition and emotional behavior, all work that is being driven by strong collaborations among the Nash Family Center for Advanced Circuit Therapeutics (CACT), the Center for Neuromodulation, the Bonnie and Tom Strauss Movement Disorders Center, and the Epilepsy Program within The Friedman Brain Institute.
Unique inferences from these studies in humans are also highly integrated with aligned research in rodents and nonhuman primates (see Cells and Circuits). In essence, observations made in humans are investigated in animals to provide insights into the underlying mechanisms, knowledge that can be brought back to the clinic to help inform new interventions in humans.
In some studies, the same cognitive tests are used in monkeys and in humans, providing an unusually powerful example of bidirectional translation. This innovative research program, integrated across several departments, several species, several neuropsychiatric syndromes, and several levels of analysis, is providing new windows into how the human brain functions and is revealing new sites to consider for future neurostimulation treatments of CNS disease.
The Living Brain Project
In addition to high-resolution imaging and precise electrode measurements, a deeper molecular analysis of brain tissue is key to achieving a more complete understanding of the human brain.
We have established a platform to safely biopsy the prefrontal cortex of …
… hundreds of living people solely for research purposes.
A long-standing barrier to progress in neuroscience is the lack of availability of living human brain tissue for molecular and cellular research. Postmortem tissue, which is fixed or frozen and lacks all function, is the standard tissue source used in such studies of the human brain, but this work is confounded by the long and variable postmortem intervals (many hours to days) between an individual’s death and procurement of brain tissue for investigation.
The Living Brain Project in The Friedman Brain Institute has established a platform to safely biopsy the prefrontal cortex of hundreds of living people solely for research purposes. For example, during deep brain stimulation surgery, implantation of electrodes involves the removal of small (several milligram) pieces of cerebral cortex that are normally discarded, which, at Mount Sinai with the patient’s consent, can now be preserved for research. Analysis of this invaluable tissue is already providing unique information about transcriptional regulation in the living human brain (see Figure 3).
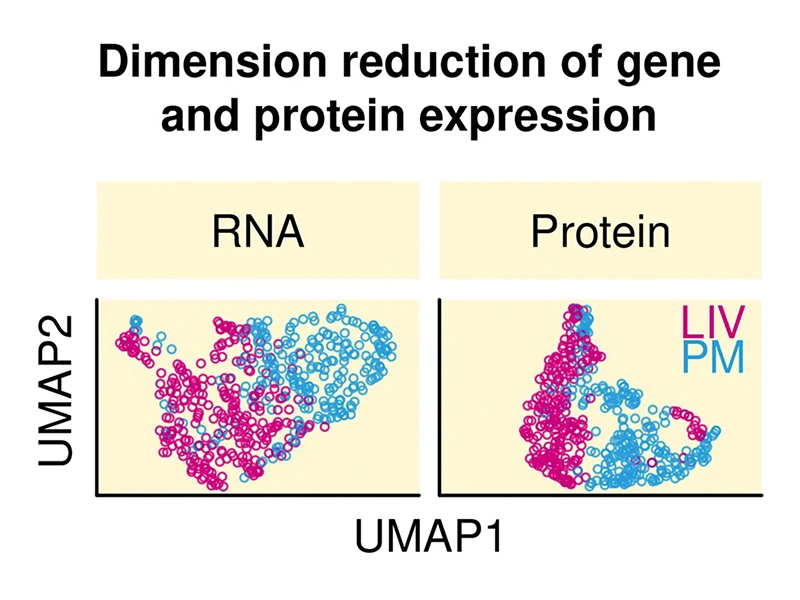
Figure 3. Scatter plots showing marked differences between living human brain samples (LIV, pink points) and postmortem human brain samples (PM, blue points) with respect to RNA expression (left scatter plot) and protein expression (right scatter plot). Expression data were reduced to two dimensions using a UMAP dimension reduction algorithm. UMAP1, which is the first dimension of the data (the variable that explains the most variance in expression) creates a near complete separation of LIV from PM samples. These findings underscore the importance and impact of having access to living human brain tissue.
Each year, more than 10 million individuals undergo neurosurgery globally, many of whom have secondary neurological or psychiatric diagnoses that are not the reason for the surgery. Prioritizing the study of living brain from these patients will expand the scope of neuroscience to include questions that can only be addressed in the context of studying living people, such as What happens at the molecular and cellular levels in the human brain as people think, feel, and act?
Our strategic collaborations among neurologists, neurosurgeons, psychiatrists, psychologists, and basic neuroscientists throughout Mount Sinai have created a “human brain laboratory” that is now yielding essential insights into the basis of CNS diseases that will increasingly drive better diagnostic tests and more effective treatments.
Featured

Priti Balchandani, PhD
Professor of Diagnostic, Molecular and Interventional Radiology, Neuroscience, and Psychiatry; Director, Advanced Neuroimaging Research Program

Ignacio Saez, PhD
Assistant Professor of Neuroscience, Neurosurgery, and Neurology, Icahn School of Medicine at Mount Sinai

Alexander Charney, MD, PhD
Associate Professor of Genetics and Genomic Sciences, Psychiatry, Neuroscience, and Neurosurgery; Co-Director, Charles Bronfman Institute of Personalized Medicine; and Executive Director, Jeff and Lisa Blau Adolescent Consultation Center for Resilience and Treatment