Faced with a patient who presents with telltale signs of HPV-related head and neck cancer, Mount Sinai’s Eric Genden, MD, has at his disposal a new technology in addition to the standard tools for diagnosis and treatment. He is increasingly opting to confirm his suspicions with a new tool that he has added to his armamentarium: an assay that is able to detect circulating tumor tissue-modified viral (TTMV)-HPV DNA, often referred to as a liquid biopsy.
“With a simple blood draw, we are able to confirm our suspicions of HPV-related disease, which is invaluable given the current HPV epidemic we are experiencing,” says Dr. Genden, the Isidore Friesner Professor and Chair of Otolaryngology – Head and Neck Surgery at the Icahn School of Medicine at Mount Sinai. “In fact, we are able to detect HPV-associated disease often months before it manifests clinically. This technology is fast becoming a major player in the way we diagnose, treat, and monitor patients.”
Developed by Naveris, the assay, NavDX®, is a unique, clinically validated TTMV-HPV DNA blood test that enables detection of HPV-driven cancer. Its proprietary technology quantifies fragments of TTMV HPV DNA, the biomarker that HPV cancer cells shed into the blood stream, and it is able to distinguish TTMV-HPV DNA from non-cancerous sources of HPV DNA. It is indicated for use with HPV16 and HPV18—the two most common high-risk HPVs—and for HPV33 and HPV35.
“The company asked us to collaborate with them in 2020 about using the assay based on the high volume of patients we see with HPV-related cancer,” says Marshall Posner, MD, Director of Head and Neck Medical Oncology; Professor of Medicine (Hematology and Medical Oncology) at Icahn Mount Sinai; and Co-director of the Protocol Review and Monitoring Committee of The Tisch Cancer Institute at Mount Sinai.
“We have been able to determine in many patients with HPV-associated throat cancer that this liquid biopsy is reliable and accurate in detecting disease, confirming the effectiveness of treatment, and helping to monitor patients for recurrent disease.”
In addition to its value as a diagnostic tool, NavDX has also been helpful in assessing the efficacy of ablative procedures in treating cancer patients. In patients who demonstrate elevated pretreatment TTMV-HPV DNA values, a decline in TTMV-HPV DNA following surgery, radiation, or chemotherapy indicates successful treatment of the tumor. Persistently elevated assay values are indicative of a persistent or incompletely treated tumor.
Dr. Genden cites one case involving the resection of an HPV-related neck tumor as illustrative of the assay’s value. Post-surgery, the patient’s titer was zero, but an assay conducted three months post-treatment was positive for TTMV-HPV DNA and the value increased in a subsequent assay. A positron emission tomography/computed tomography scan revealed a small area of cancer on the opposite side of the patient’s neck that had not been identified.
“This is a patient whose disease may have gone undetected until it became advanced and necessitated debilitating life-changing radiotherapy for treatment,” Dr. Genden says. “By following the assay numbers, we have a reliable guide as to whether we have removed the entire tumor, whether it has recurred, or whether there is further disease that was not encompassed by therapy.”
Graph: The patient presented with locally advanced HPV head and neck cancer—pretreatment liquid biopsy was positive.
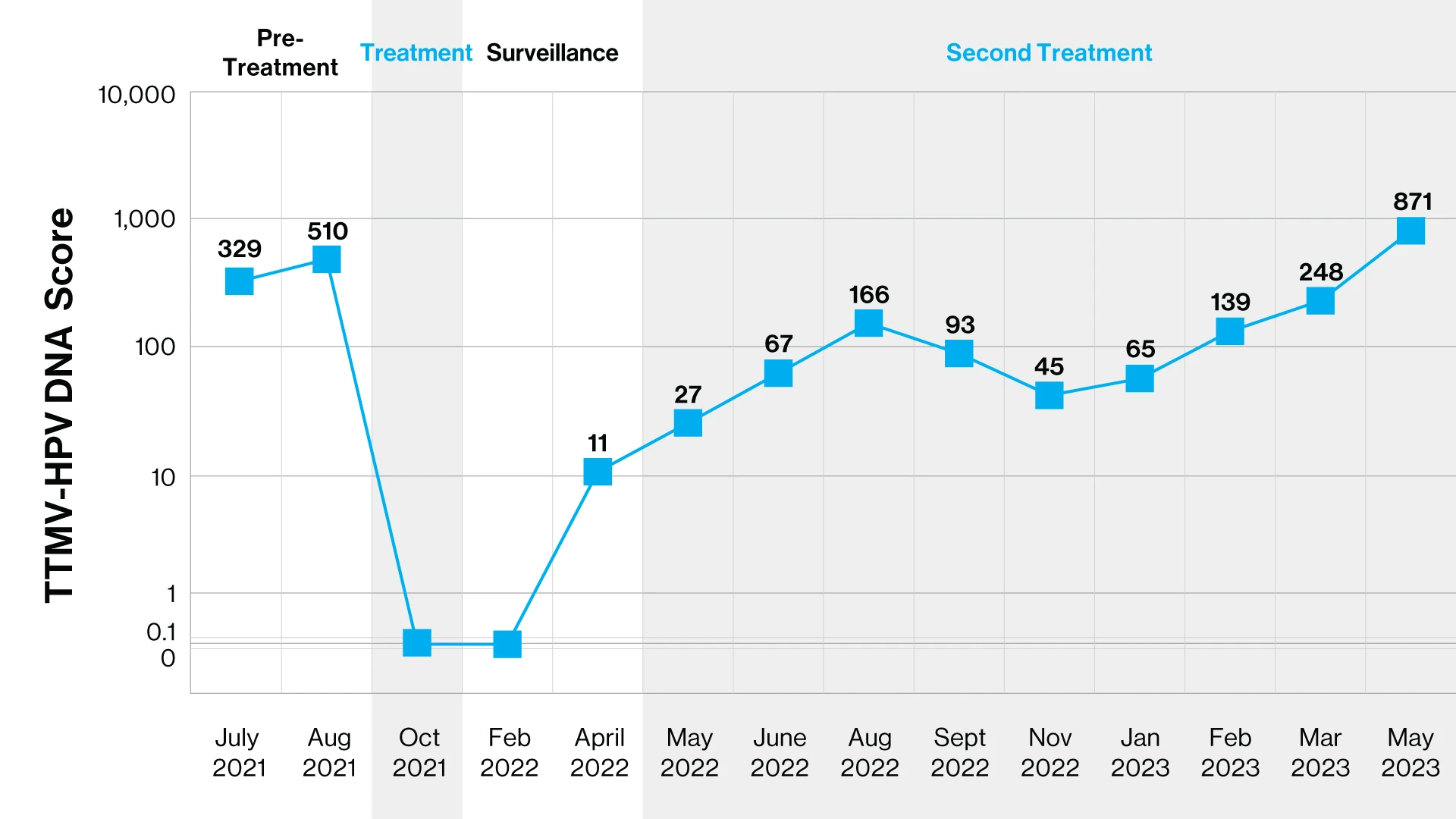
The patient was treated with chemotherapy and chemoradiotherapy with clinical and liquid biopsy complete response. Six months later, liquid biopsy became positive; imaging was negative. Four months later, lung metastases were identified. Immunotherapy was started and a partial response by liquid biopsy and imaging lasted four months. Liquid biopsy increased; PET scan was stable until imaging progression after nine months. Patient was then started on another therapy.
That said, Dr. Posner cautions that the assay is associated with a certain degree of false negatives—approximately 5 percent based on their data—and they are investigating this phenomenon further to assess the associated risk. He hypothesizes that this is the result of the low number of TTMV-HPV DNA released by some of the tumors, either due to biology or low volume of cancer or both. Moreover, he adds that the assay is not as effective in assessing response to immunotherapy or vaccine treatment modalities.
“The fluctuation in the numbers is too great at this time for us to determine whether the assay is accurately reflecting whether cancer is responding, stable, or growing,” Dr. Posner says. “You can follow a general trend, but you cannot get a precise number. That may be because there is cytolytic or immune-modulated cell death that is increasing or decreasing the amount of apoptotic cell death. It is possible the assay is actually more accurate then scans, however, this is where our research is investigating the utility and biology of the assay.”
These are some of the questions Dr. Posner hopes to gain more insights on with further data. He is also looking to gain more insights on prediction of recurrence, specifically whether he and his colleagues should be looking at absolute or relative numbers to assess that risk. Meanwhile, Dr. Genden is studying whether negative titers post-surgery can be used to rule out radiotherapy, which would significantly reduce treatment-associated morbidity among patients. Regardless, Drs. Genden and Posner agree the assay has already proven to be invaluable.
“The bottom line is we now have a tool that enables us to make determinations that improve patient outcomes and morbidity with less inconvenience, fewer side effects, and less cost,” Dr. Posner says.
Featured

Eric M. Genden, MD, MHA, FACS
Professor and Chair of Otolaryngology – Head and Neck Surgery

Marshall Posner, MD
Director, Head and Neck Medical Oncology; Professor, Medicine (Hematology and Medical Oncology); Co-director, Protocol Review and Monitoring Committee, Tisch Cancer Institute