Transplantations involving the trachea or the lungs are challenging in terms of suitability and patient outcomes. However, research conducted by Ya-Wen Chen, PhD, could one day eliminate such complications.
“Our idea is that if we can take cells from a patient, reprogram them back to stem cells, and then use those cells to grow airway stem cells or lung stem cells to transplant them back to the patient, that might reduce the immune rejection or lower the requirement for immune suppression,” says Dr. Chen, Assistant Professor of Otolaryngology, and Cell, Developmental, and Regenerative Biology, at the Icahn School of Medicine at Mount Sinai. “There is also the potential that we could use these cells to repair the trachea or the lungs, eliminating the need for transplantation altogether.”
Dr. Chen could achieve those aims, and a better understanding of the molecular drivers of respiratory diseases, through her work with human pluripotent stem cells (hPSCs). These cells, which include embryonic stem cells and induced pluripotent stem cells (cells from an adult that have been reprogrammed back to stem cells), have the potential to develop into any cell or tissue in the body. Using directed differentiation, a bioengineering methodology in which hPSCs are given specific developmental instructions through growth factors or small molecules, Dr. Chen is harnessing that potential by attempting to mimic the necessary in vivo signaling to grow everything from basal cells to lung organoids.
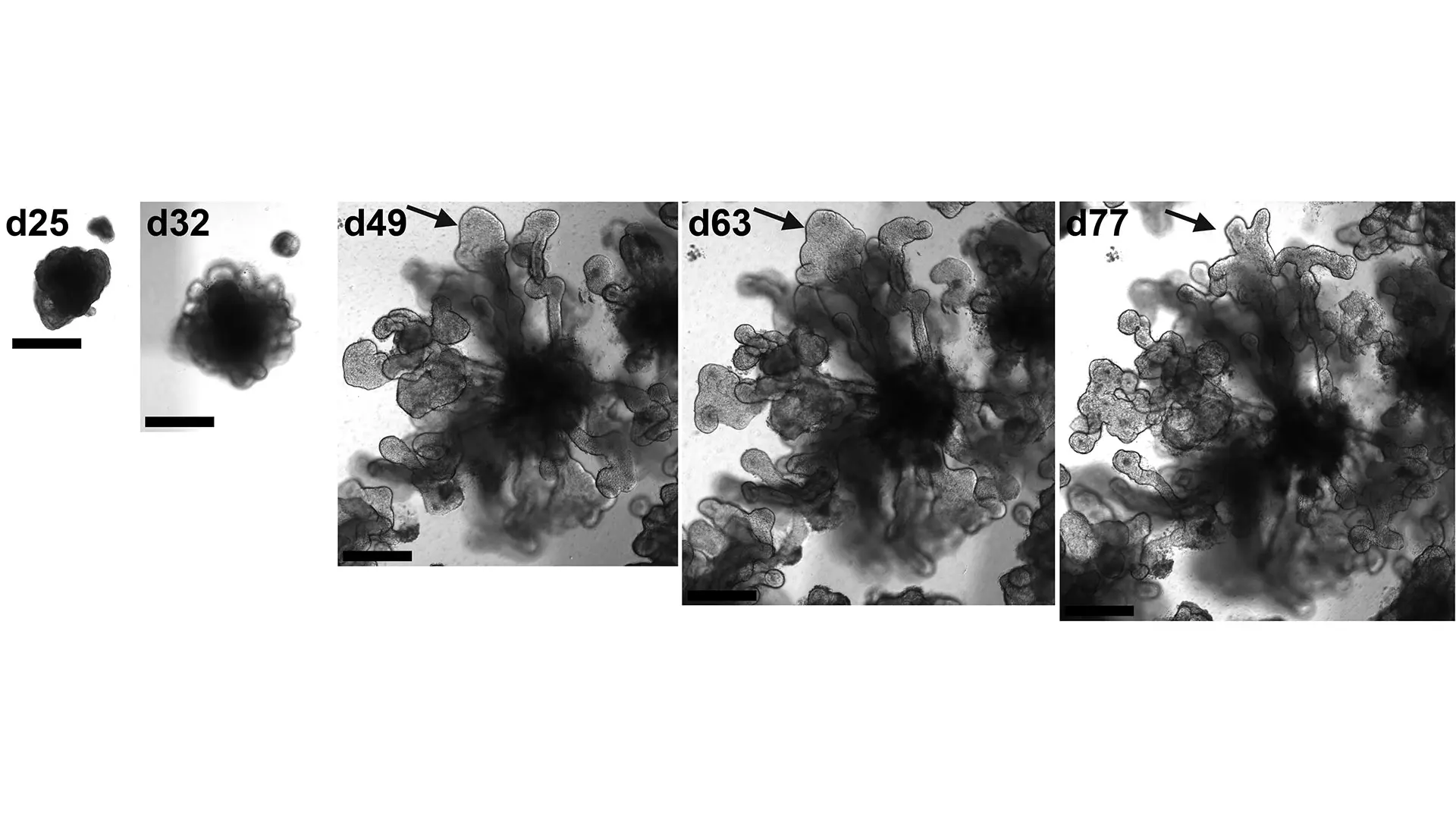
Bright field images of the development of a lung organoid into a branching structure. The arrow indicates a branching tip that is bifurcating.
“We developed the first lung organoid model using hPSCs, which we can grow in a dish and transplant into a mouse, that has part of the lung structure performing part of the lung function,” says Dr. Chen. “We are using the organoid model to do modeling of diseases such as COVID-19, influenza, and fibrosis, so that we can understand how these diseases were initiated, and conduct drug screening to help us identify preventive or therapeutic agents. We are also using the model to explore lung development, mechanisms of lung injury repair, and regenerative medicine approaches.”
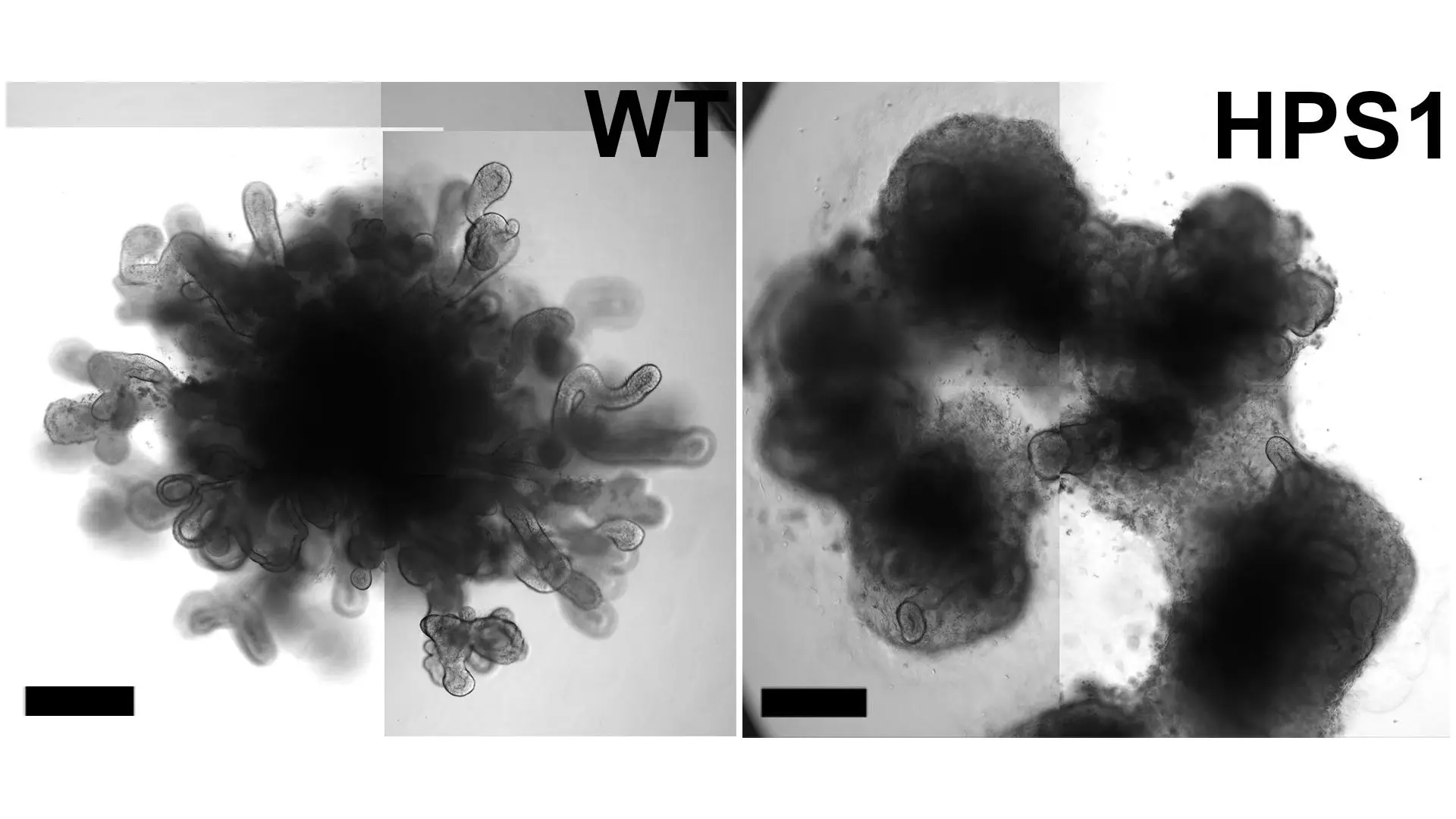
Bright-field images showing day 50 lung organoids without (WT) and with (HPS1), a mutation that would cause fibrosis.
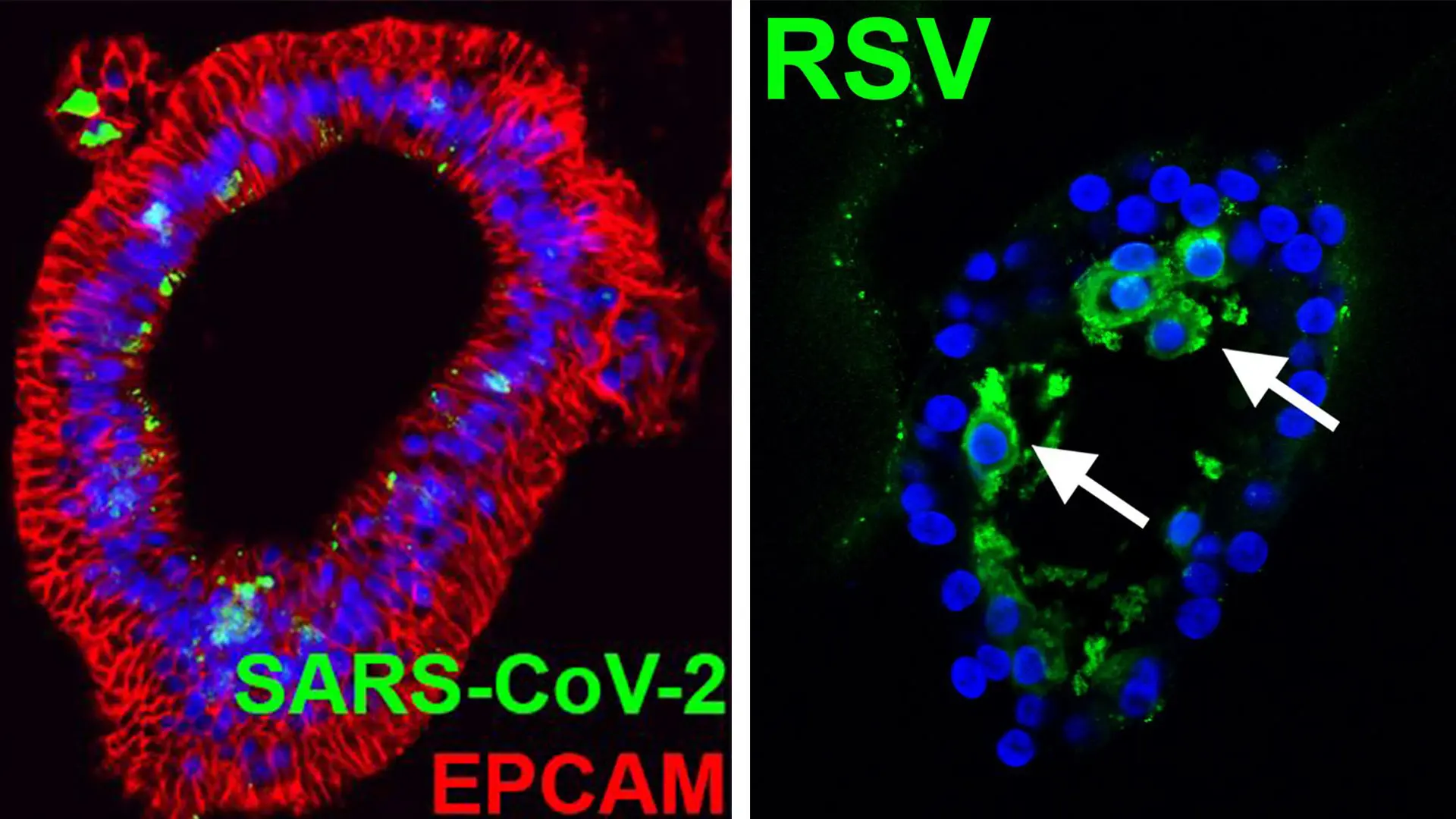
Confocal images of lung organoids infected with SARS-CoV-2 (NP) or RSV (arrows: infected cells in the lumen).
Could Stem Cells Eliminate the Need for Transplantations Entirely?
The potential of Dr. Chen’s work is exemplified by a partnership with the Institute of Airway Sciences at Mount Sinai that is assessing the efficacy of using hPSCs to generate and expand basal cells to reconstruct the trachea’s epithelial cells, and thus the trachea. This method could result in patients receiving a trachea transplant with epithelial cells that have been bioengineered from their own cells rather than relying on a transplant from a donor. This could mitigate the risk of immune rejection, or lower the requirement for administration of immunosuppressants. But it is also possible that direct delivery of these stem cells to the airway could eliminate the requirement for transplantation among certain patients.
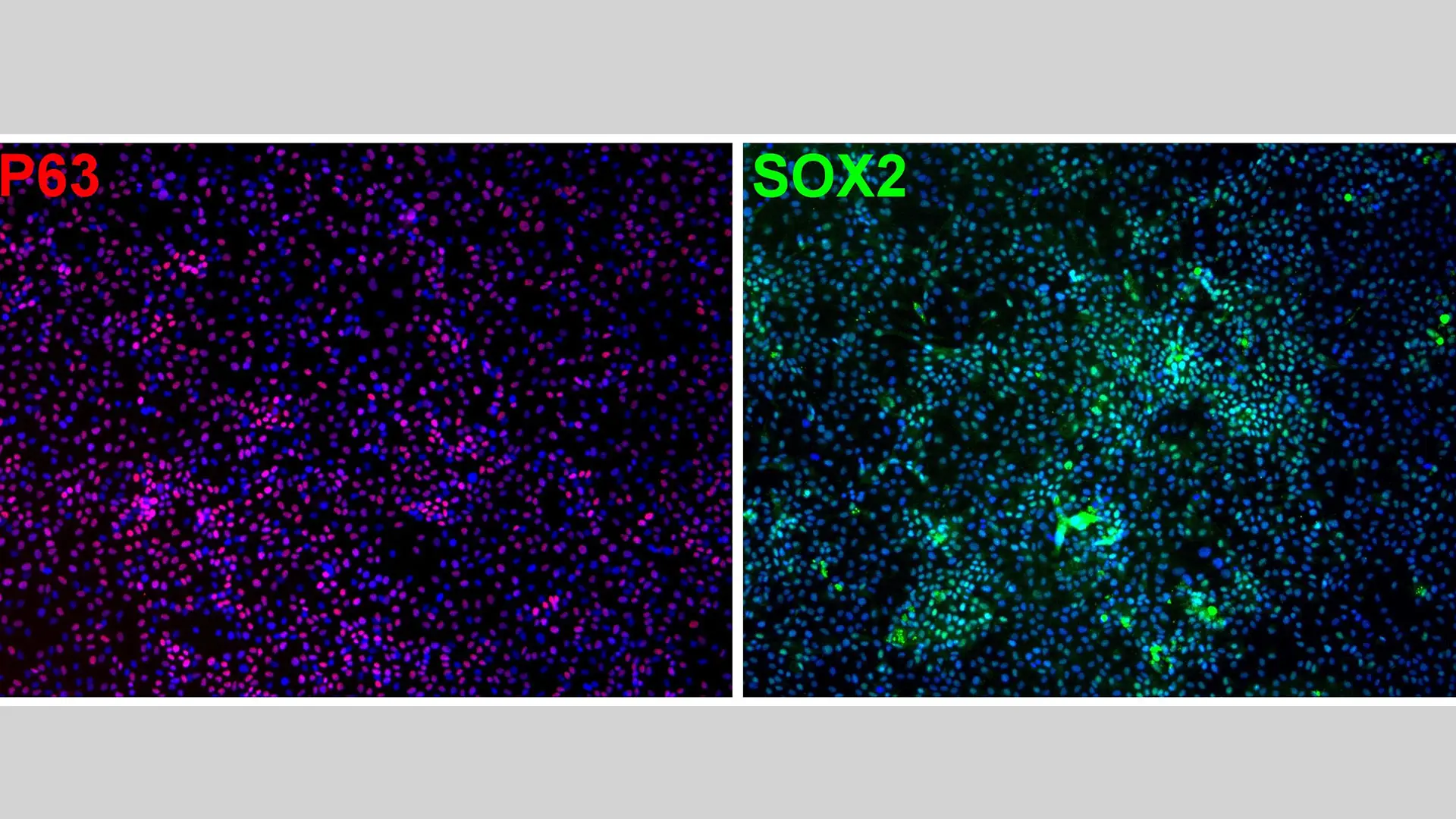
Immunofluorescence staining of markers expressed in basal cells.
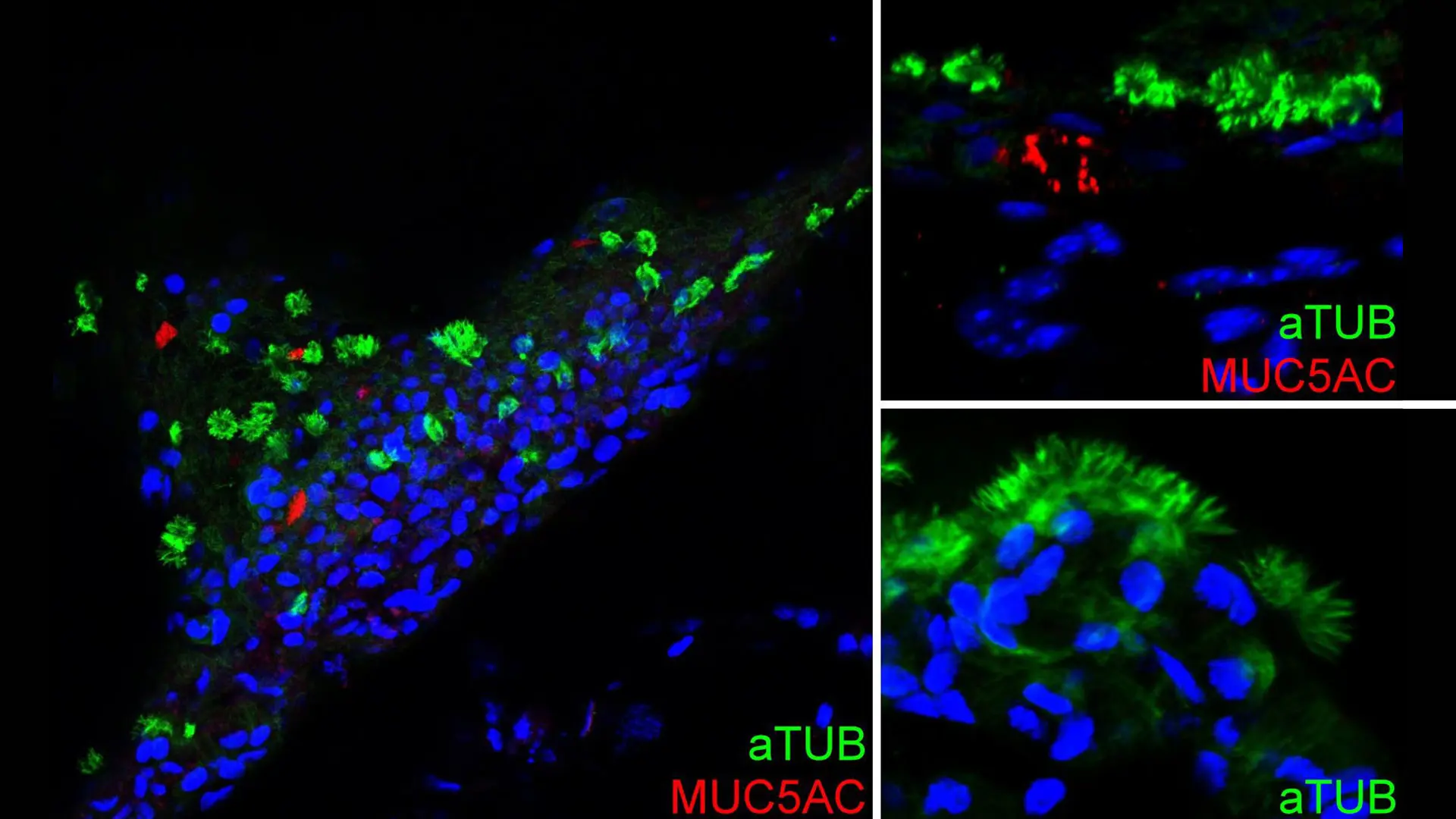
Immunofluorescence staining of hPSC-basal cells after two months of air-liquid interface culture showing differentiation of basal cells into goblet cells (MUC5AC) and ciliated cells (aTUB).
“We will be exploring this first in an animal model,” Dr. Chen says. “We will remove the epithelial cells in the trachea, apply the cells to the site, and then monitor them to see whether they attach, survive, proliferate, and, most important, differentiate into the right cell types at the right location to perform the correct functions. We know that they differentiate when we culture them in the lab environment, but we do not know how they will behave in animals or in humans.”
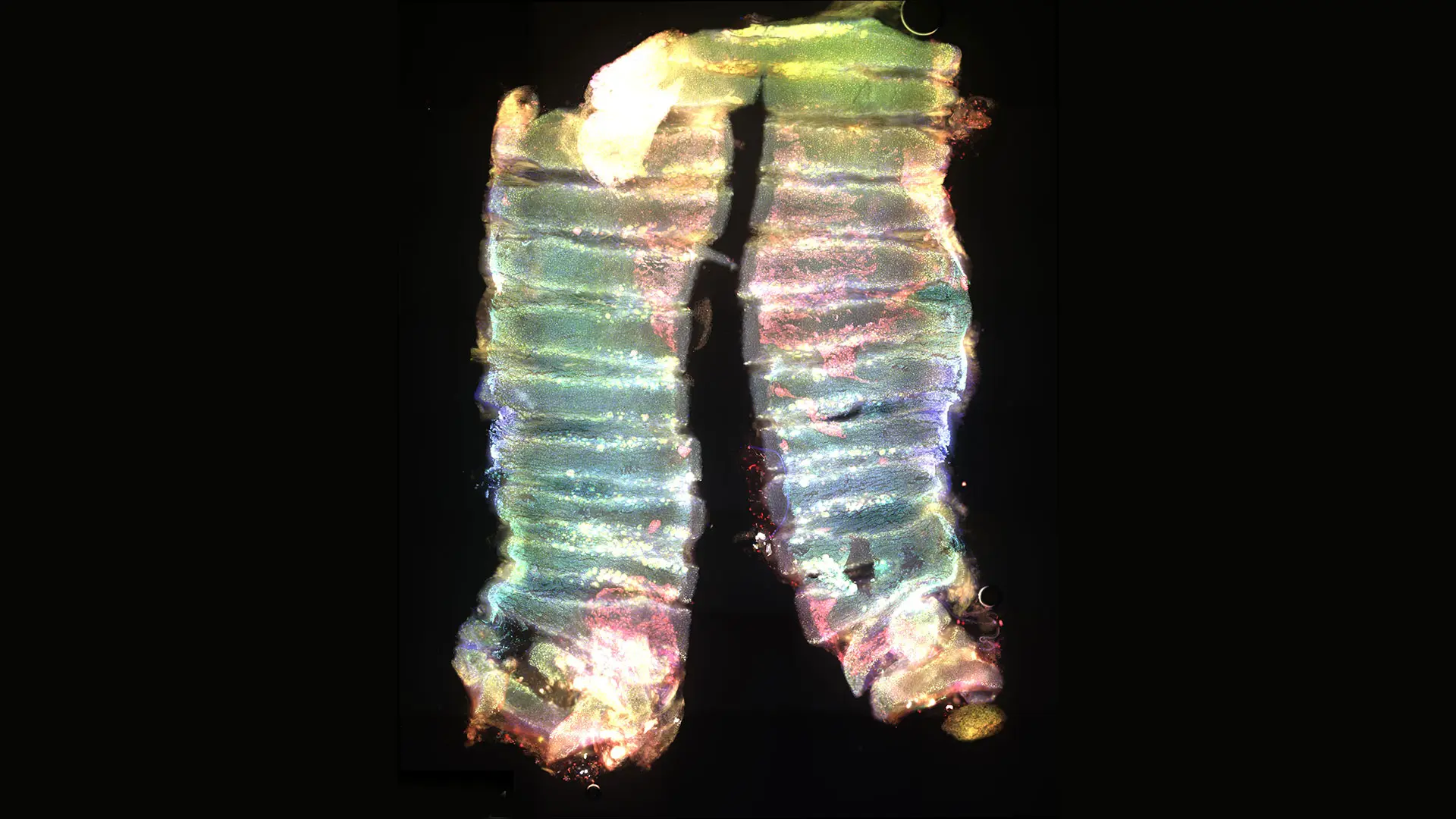
Immunostaining of human-specific markers (red) showing transplanted hPSC-basal cells engrafted and expanded on the luminal surface of a de-epithelial mouse trachea.
Gaining Better Insight into SARS-CoV-2
Dr. Chen’s research is also advancing our understanding of the mechanisms by which SARS-CoV-2 enters the lungs. Using her lung organoid model, she is assessing the potential of using antibodies to block the virus from using transmembrane serine protease 2 (TMPRSS2), a protease used by the virus to enter cells. She will first test whether TMPRSS2 antibodies are toxic to the lung cells she has grown and, once she has established a safe dosage, assess their ability to block the virus. She will then investigate whether that blockage occurs through reduction of TMPRSS2 on the surface of the lung cell or by blocking the function of TMPRSS2. The outcomes of these inquiries will be validated with lung organoids and animal models.
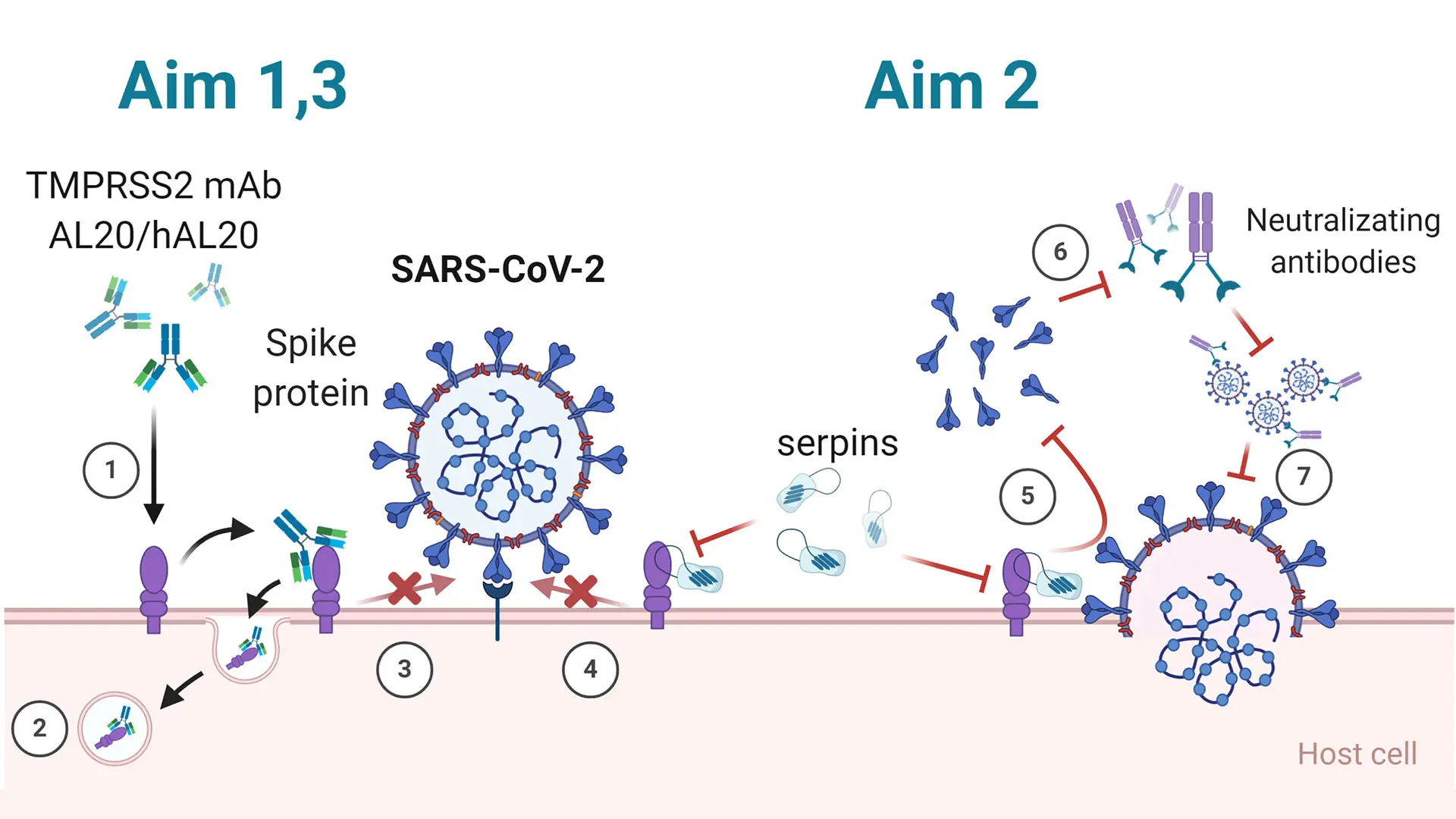
Caption: TMPRSS2 primes the S protein, permitting SARS-CoV-2 cellular entry and spread. Aim 1: 1) AL20 blocks TMPRSS2. 2) Binding of AL20 to TMPRSS2 induces endocytosis and reduces cell surface TMPRSS2. 3) Binding of AL20 inhibits TMPRSS2 activity and blocks viral entry. Aim 2: 4) Binding of serpins abolishes TMPRSS2 activity and blocks viral entry. 5) Loss of TMPRSS2 activity decreases shedding of S protein. 6) Shed S proteins bind to neutralizing antibody inhibiting its ability to block viruses. 7) Viruses bound by neutralizing antibody cannot bind to ACE2 receptor. Aim 3: 1) Humanized AL20 blocks SARS-CoV-2.
“Our findings could impact the lives of millions of people who have been affected by COVID-19 and other respiratory viruses, such as influenza A, that use TMPRSS2 to enter cells,” says Dr. Chen. “Furthermore, given that most of the coronavirus family relies on TMPRSS2 to infect lungs, our findings could also provide a means of defense against the next coronavirus outbreak.”
The Potential to Generate a Human Lung Using hPSCs
These undertakings would be impactful, but Dr. Chen has ambitions that go beyond what is currently achievable in the realm of hPSCs. She hopes to recapitulate a human lung ex vivo using a patient’s hPSCs for possible transplantation, which would help resolve the suitability, shortage, and quality issues related to organ donation. But she also envisions regenerating the lung in vivo, which could eliminate the need for transplantation.
“Again, we know we can generate lung organoids in the lab, transplant those into a mouse, mimic human lung development, and then generate mini lungs,” Dr. Chen says. “To do that in a human patient, we need to increase our stem cell population and scale up our techniques. Even so, we do not know if signals from the injured site will stimulate the stem cells to regrow the lung, but my goal is to see if that is possible.”
Featured

Ya-Wen Chen, PhD
Associate Professor of Otolaryngology, and Stem Cell Biology and Regenerative Medicine