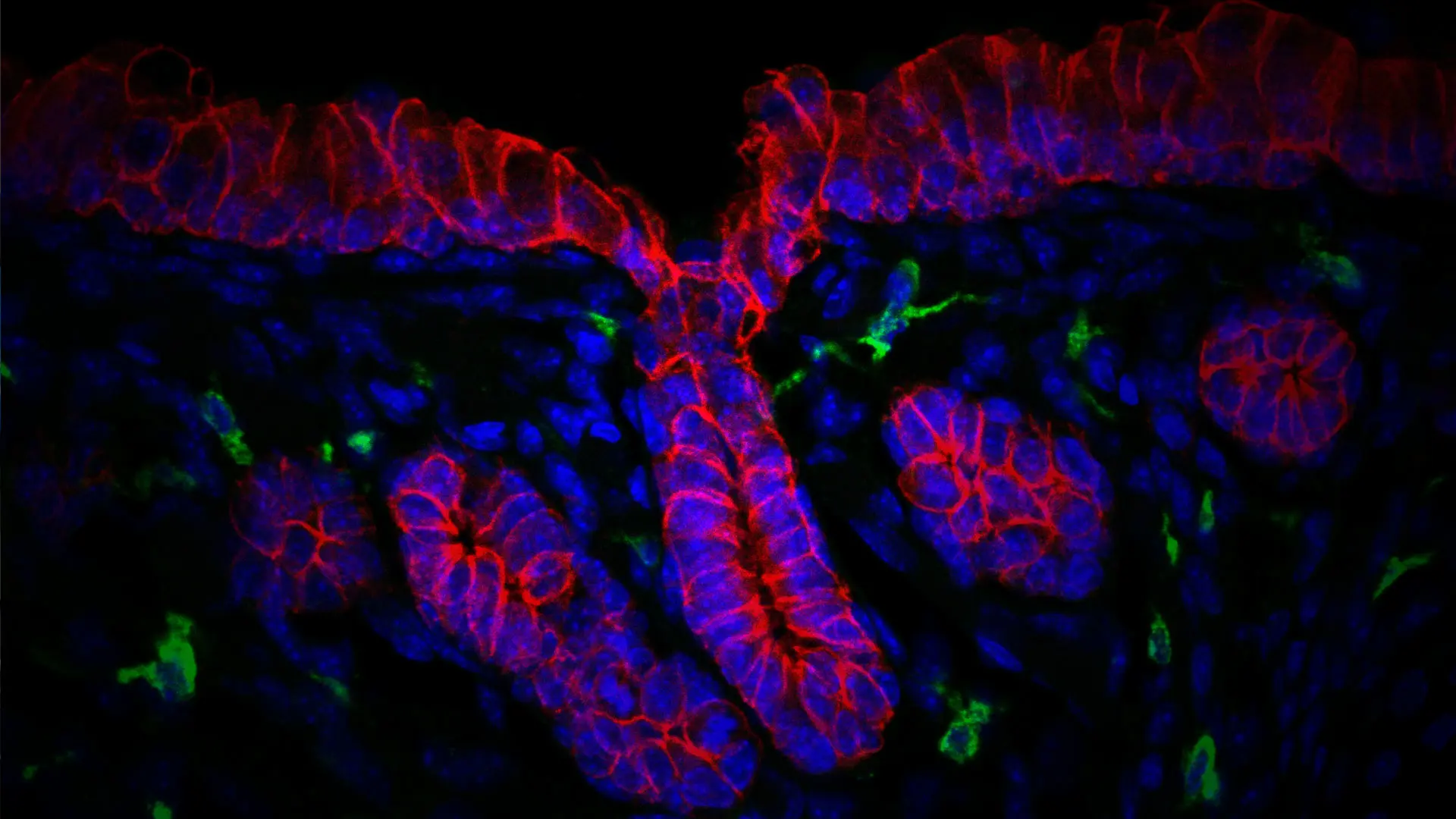
The respiratory submucosal glands (SMG) play a crucial role in protecting the lungs from infection by producing mucus that traps harmful organisms from the environment. But Mount Sinai’s Alison May, PhD, says that is not the case among patients who have diseases such as cystic fibrosis.
“With these patients, we typically see abnormally functioning SMGs that secrete thickened mucus that cannot be cleared, in the upper airway in the nose and sinuses, as well as the distal lung,” explains Dr. May, Assistant Professor of Cell, Developmental, and Regenerative Biology, and Otolaryngology, at the Icahn School of Medicine at Mount Sinai.
“The result,” she continues, “is infection and an environment for these organisms to thrive, resulting in chronic facial pain and lung disease, and a continual cycle of triggering an inflammatory response.”
Dr. May may be on the verge of breaking that cycle. Supported by the Innovation Award from the American Lung Association, her lab is investigating how tissue-resident macrophages—immune system cells—regulate the formation of SMGs. Through her research, Dr. May observed the recruitment of innate immune cells to the airway in parallel with the formation of the glands.
“That indicates that these immune cells may play some role in guiding the development of the glands,” Dr. May says. “The question for us now is, are these immune cells necessary for that process?”
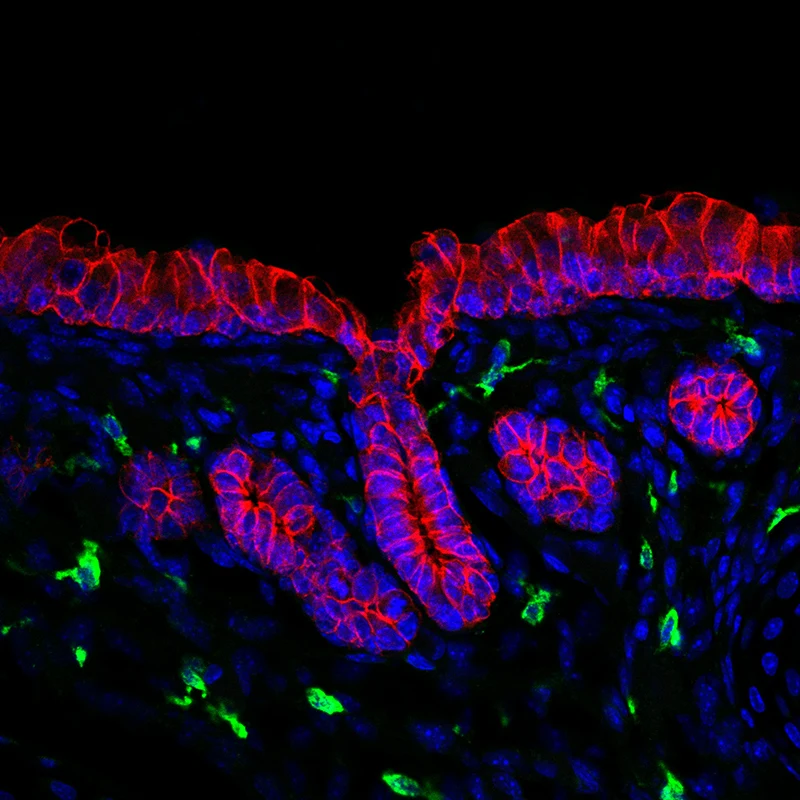
Immune cells (green) of the airway glandular niche. Gland and airway epithelium (outlined in red), nuclei of cells (blue).
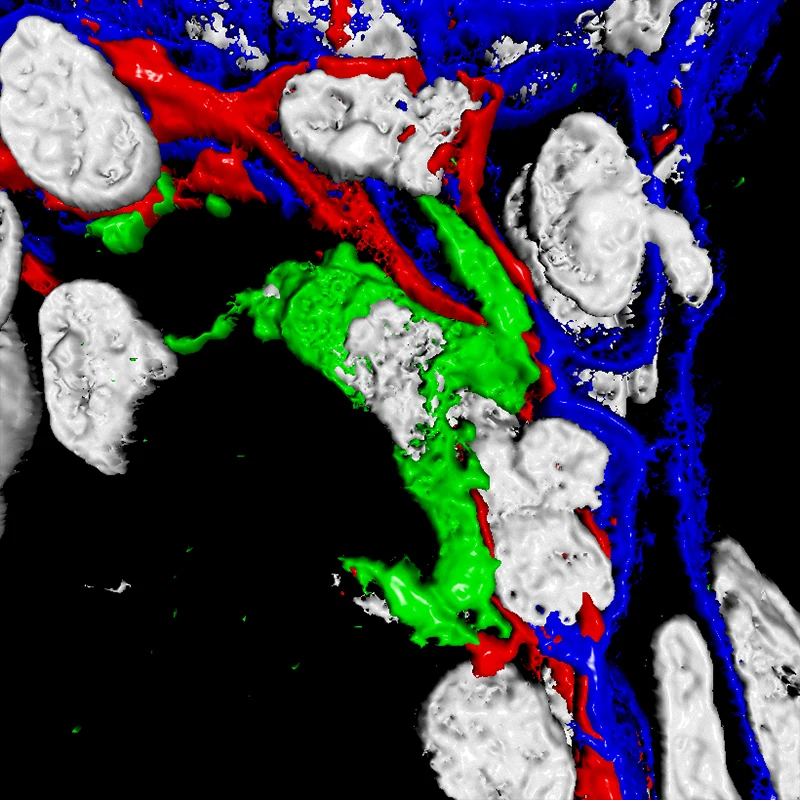
3D image rendering of an immune cell (green) interacting with heterogenous glandular cells (red and blue). Nuclei of cells (white).
Gaining Valuable Insight Into the Interaction Between Macrophages and SMGs
Dr. May and her team are using a combination of animal models, lab-grown organ explants, tissue biopsies, and computational biology to look at the interactions, or cross talk, that occur between macrophages and SMGs in the development and maintenance of healthful glands. She is also exploring how those interactions regulate the mechanisms needed to establish different cell types.
“We want to know what messages are being sent during the formation of healthful glands and, when that gland is formed, what are the messages that maintain that environment,” she says. “Once we establish that, we can start understanding how this goes awry in disease states.”
This is the long-term goal of Dr. May’s research. By identifying signals sent by a macrophage to an epithelial cell that cause it to divide in a way that is fundamental for normal development of the glands, she can assess whether these signals are upregulated in a disease state, thus resulting in larger or more glands.
“We are essentially defining the developmental role of these cells, and the precise interactions and time points that determine what they are going to be, which is a very innovative approach,” Dr. May says. “This will also give us great insight into how developmental mechanisms are disrupted in cystic fibrosis, giving rise to early onset of aberrant gland morphology and function.”
As the interactions among immune cells and glands become clearer, Dr. May will engage the Department of Otolaryngology − Head and Neck Surgery and the Institute for Airway Sciences at the Icahn School of Medicine at Mount Sinai to further explore the mechanisms at play, identify targets for treatment, and potentially develop therapeutics for glandular repair in airway diseases. This, she notes, is where cell and organ cultures will be invaluable.
“We need to test our targets and their impacts on other cells,” she says. “We want to ensure they do not indirectly affect other cell types through some other mechanism. Ultimately, I am optimistic that, through our work, we can reduce harmful signals, and promote helpful signals, from the immune cells to the glands and develop long-lasting therapeutic avenues for airway disease.”
Featured

Alison May, PhD
Assistant Professor of Cell, Developmental, and Regenerative Biology, and Otolaryngology