Researchers from the Diabetes, Obesity and Metabolism Institute at Mount Sinai have discovered a novel therapeutic strategy to protect insulin-producing beta cells from the damaging effects of glucolipotoxicity—a major contributor to the progression of type 2 diabetes. The findings were published in March 2025 in Nature Communications.
“For the first time, we’ve shown that it’s possible to use small molecules to fine-tune carbohydrate response element binding protein (ChREBP) activity in a way that could have major therapeutic implications,” said lead author Liora S. Katz, PhD, Associate Professor of Medicine (Endocrinology, Diabetes and Bone Disease) at the Icahn School of Medicine at Mount Sinai. “This is an exciting step forward in our understanding of beta cell protection and the prevention of diabetes deterioration.” For patients, the research could lead to new treatments that protect the insulin-producing cells in the pancreas, potentially slowing or even preventing the progression of diabetes.
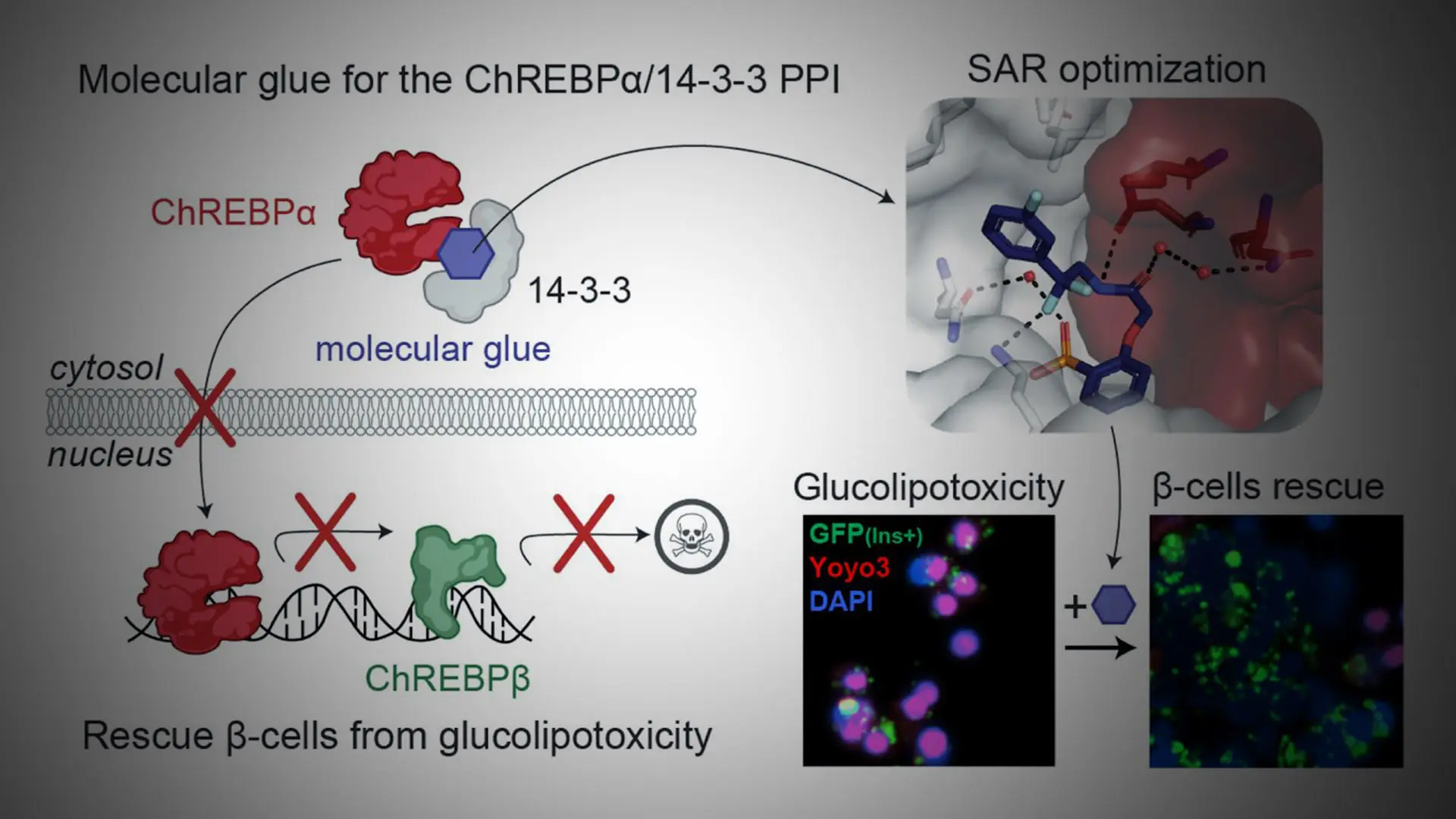
ChREBP is a key transcription factor that plays a crucial role in regulating glucose metabolism. It exists in two main isoforms: ChREBPα and ChREBPβ. Under conditions of glucolipotoxicity, ChREBPα goes into the nucleus and starts making too much ChREBPβ, which contributes to beta cell dysfunction and death.
The Mount Sinai team, in collaboration with researchers from Eindhoven University of Technology and the University of Duisburg-Essen, identified and developed small molecules—termed “molecular glues”—that enhance the interaction between ChREBPα and 14-3-3 proteins. These glues stabilize ChREBPα in the cytoplasm, preventing its nuclear entry and the subsequent overproduction of ChREBPβ.

Illustrating the mechanism by which small molecule stabilizers of the ChREBPα/14-3-3 protein-protein interaction (PPI) protect insulin-secreting beta cells from glucolipotoxicity. The optimized "molecular glue" compounds retain ChREBPα in the cytoplasm, preventing its transcriptional activity and subsequent beta cell dedifferentiation and death. This approach highlights a novel therapeutic strategy for maintaining beta cell identity and function in the context of type 2 diabetes.
When tested in primary human beta cells, these molecular glues significantly reduced the toxic effects of glucolipotoxicity, thus preserving beta cell function and identity. This discovery represents a major shift in diabetes research, as transcription factors like ChREBP have long been considered "undruggable" targets. “This study may thus not only provide the basis for the development of a unique class of compounds for the treatment of type 2 diabetes but also showcases an alternative ‘molecular glue’ approach for achieving small molecule control of notoriously difficult to target transcription factors,” the study concluded.
“Our findings suggest a completely new strategy for preserving beta cell function in diabetes,” said Donald K. Scott, PhD, Professor of Medicine (Endocrinology, Diabetes and Bone Disease) at the Icahn School of Medicine. “This approach could complement existing diabetes treatments and help prevent disease progression.”
Researchers are now working to refine these compounds and evaluate their potential for clinical translation. Future studies will focus on optimizing the molecular glues for therapeutic use and testing them in preclinical diabetes models.
The work was supported in the United States by NIH/NIDDK R01DK130300 and the Human Islet and Adenoviral Core (HIAC) of P30DK020541, and in the European Union through ERC Advanced Grant PPI-Glue (101098234), the Netherlands Ministry of Education, Culture and Science (Gravity program 024.001.035), the Netherlands Organization for Scientific Research (ECHO grant 711.018.003), and by DFG-funded CRC1093 (Supramolecular Chemistry on Proteins).
“This beautiful study represents the culmination of several decades of work by the Katz-Scott team and their European collaborators. It deepens our understanding of the remarkably complex ChREBP alpha and beta transcription factor system, and how it functions to alternately drive—or protect against—the loss of insulin-producing pancreatic beta cells in response to excessive nutrient burdens,” says Andrew F. Stewart, MD, Director of the Diabetes, Obesity and Metabolism Institute, and Professor of Medicine (Endocrinology, Diabetes and Bone Disease), Icahn School of Medicine. “The identification of novel 'molecular glues' as future drug candidates that can mitigate beta cell loss in type 1 and type 2 diabetes is a remarkable achievement and predicts new treatment paradigms for these devastating diseases.”

Donald Scott, PhD, and Liora Katz, PhD