Among the many difficult decisions facing endocrinologists monitoring feminizing gender-affirming hormone therapy (GAHT) is striking a balance between serum concentrations of testosterone and estradiol that is optimally beneficial to their patients. In the most comprehensive study of its kind to date, scientists from the Mount Sinai Center for Transgender Medicine and Surgery in a 2025 study found that testosterone may serve as a more reliable marker than estradiol for monitoring treatment effectiveness, particularly in individuals with intact testes. For post-orchiectomy patients who have had their testes removed, the team reported that luteinizing hormone (LH) concentrations represent a reasonable surrogate for testosterone for monitoring GAHT. The study was published in December 2025 in Endocrine Practice.
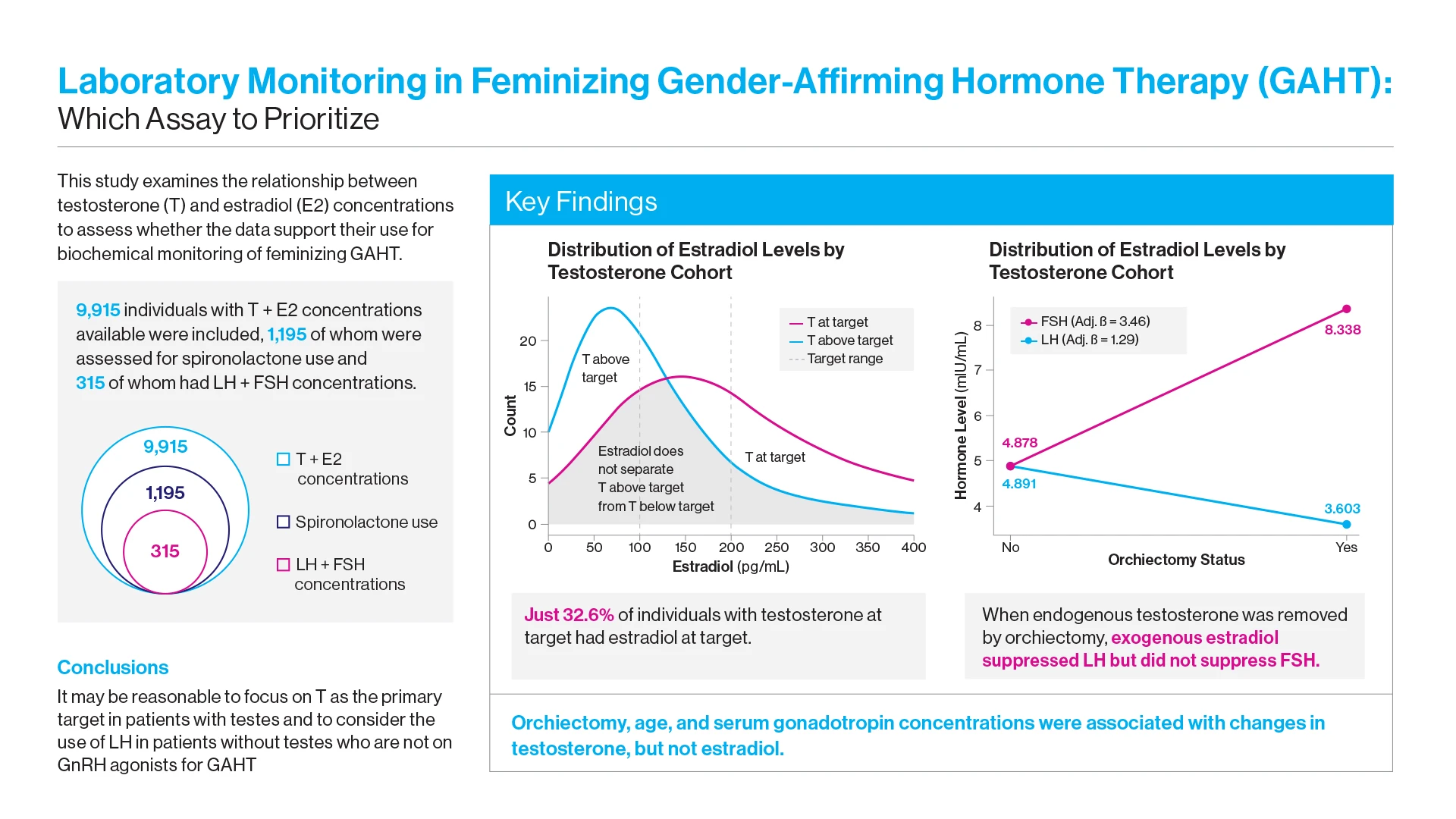
Through a retrospective analysis of nearly 10,000 GAHT individuals, investigators sought to learn how often providers were able to achieve concordance between guideline-recommended testosterone and estradiol concentrations. And in cases where they could note, did it make more sense to clinically refocus on one target versus the other, or consider using different biomarkers altogether?
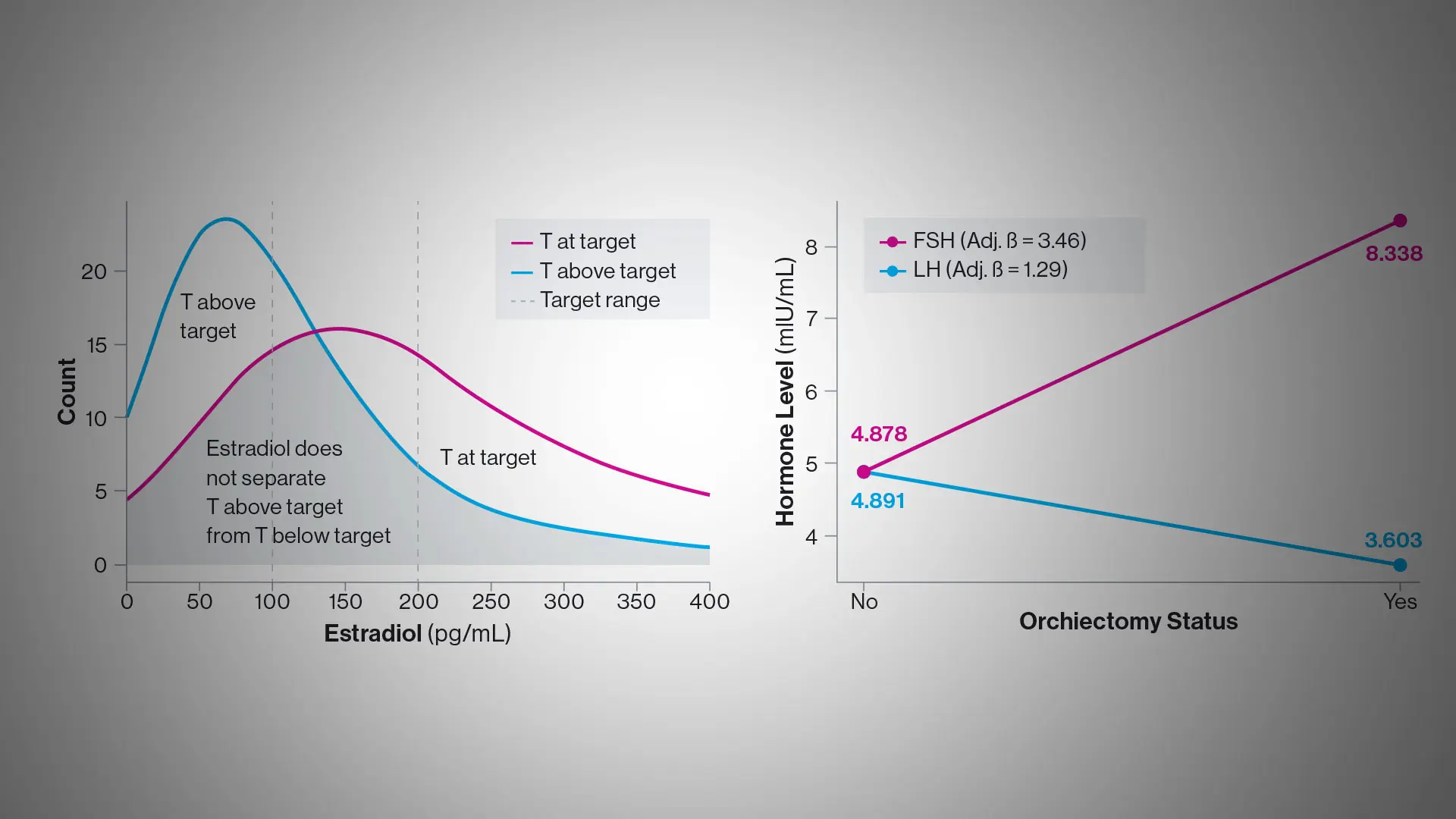
“Underscoring the complexity of the task for endocrinologists, we learned that only about a third of individuals undergoing feminizing gender-affirming therapy who had testosterone concentrations at target also met estradiol guidelines,” says Daniel Slack, MD, Assistant Professor of Medicine (Endocrinology, Diabetes and Bone Disease) at the Icahn School of Medicine at Mount Sinai, and lead author of the study. “Just as importantly, we found that serum testosterone was more consistently associated than estradiol with key clinical variables such as age, orchiectomy, and use of spironolactone, and therefore may be a more dependable and accurate marker for monitoring feminizing hormone therapy.”
Guiding endocrinologists in their choice of hormone assay for patients are the recommendations of the World Professional Association for Transgender Health (WPATH) and the Endocrine Society. Those standards specify titrating patients to testosterone concentrations below 50 ng/dL, and estradiol concentrations between 100 and 200 pg/ml. Supported by those guidelines, clinicians look to provide gender-affirming feminizing therapy that can stimulate a balance of estrogen and testosterone receptors throughout the body consistent with a typical female hormone profile.
A graphical abstract of the study in Endocrine Practice.
Researchers expressed concern, however, that a common scenario in clinical practice would be patients with testosterone concentrations at target and estradiol below target. Providers might increase the dose of estradiol for no clinical benefit while potentially increasing patient risk. Similarly, there may be individuals with testosterone at target and estradiol concentrations marginally above target where providers might lower the dose of estradiol, a decision that might be both clinically unwarranted and less likely to produce the desired feminizing outcomes.
“One of our major findings is that the field may be overly reliant on estradiol assay despite its apparent lack of associations with important clinical variables, suggesting limitations in relying on estradiol for treatment monitoring,” says senior author Joshua Safer, MD, Professor of Medicine (Endocrinology, Diabetes and Bone Disease) at the Icahn School of Medicine, and Executive Director of the Mount Sinai Center for Transgender Medicine and Surgery (CTMS). Because of its large volume of patients, as well as its leading-edge research and vast body of knowledge and expertise, CTMS is widely seen as a national leader in the field.
“We believe clinicians should instead be focused on testosterone assay as their primary marker for titration and treatment,” Dr. Safer says, “and use estradiol not so much as a standard of care but as a secondary safety check to ensure that testosterone suppression is not accompanied by very high physiologic levels of estradiol.”
At the same time, the Mount Sinai team learned that luteinizing hormone concentrations declined in tandem with testosterone, suggesting they could serve as a valid surrogate marker for treatment response in individuals whose prior orchiectomy—roughly half of all GAHT patients—removed testosterone as an active treatment marker. In such cases, estradiol remains the standard practice protocol.
“These findings suggest that estradiol assays may be most useful for identifying overtreatment, rather than serving as a primary treatment target for feminizing GAHT,” the study concluded. “Future research should prioritize prospective longitudinal studies that correlate serum hormone concentrations with both externally measured health outcomes and patient reported outcomes.”
Dr. Slack elaborates, “We think our latest work has the potential to get physicians thinking hard about the guideline recommended target ranges they rely heavily on in their practices, and whether they really make sense. Our goal now is to advance that level of inquiry and, more generally, broaden the field’s knowledge about use of appropriate hormone assays by disseminating the evidence we gathered in our study to our colleagues across the medical community.”
Featured

Joshua Safer, MD
Professor of Medicine (Endocrinology, Diabetes and Bone Disease); Director, Center for Transgender Medicine and Surgery

Daniel Slack, MD
Assistant Professor of Medicine (Endocrinology, Diabetes and Bone Disease)