A new era has emerged in the treatment of thyroid eye disease (TED), informed by years of research at Mount Sinai into the pathophysiology of this autoimmune disorder. In a significant advance in 2025, a team led by Terry F. Davies, MD, described for the first time the tight biological bond between two receptors known to factor in the remodeling of tissues in thyroid eye disease.
The findings, reported in Endocrinology could have significant implications for developing new therapies for this potentially disfiguring disease, says Dr. Davies, the Florence and Theodore Baumritter Professor of Medicine, Icahn School of Medicine at Mount Sinai, and a world leader in the study of autoimmune thyroid disease.
The new era in treatment of thyroid eye disease began in 2020, with approval by the U.S. Food and Drug Administration of teprotumumab-trbw (Tepezza®). This monoclonal antibody works by blocking the protein IGF-1R (insulin-like growth factor -1 receptor inhibitor), reducing inflammation and preventing muscle and fat tissue remodeling, the hallmarks of TED. Tepezza became the first therapy to reduce proptosis and improve care of the most difficult cases, and its approval set off efforts by drugmakers to find other interventions and intensified the search for a better mechanistic understanding of TED.
“Growing evidence shows that thyroid eye disease is due to signal enhancements in orbital fibroblasts located behind the eye as a result of the antibody-induced interaction between the thyroid-stimulating hormone (TSH) and IGF-1 receptors,” Dr. Davies says. “Through our team’s advanced modeling, we were able to show that these receptors interact with each other directly at their ectodomains. Moreover, we learned that they fit together like a combined molecule to synergize signaling capacity, though further investigation into the mechanisms of this union will be crucial to uncovering new therapeutic interventions.”
About 40 percent of patients with Graves’ disease suffer from thyroid eye disease and its potentially severe side effects of proptosis, diplopia, pain, and inflammation. TED occurs when TSH receptor antibodies mistakenly target the TSH receptor on the surface of thyroid epithelial cells. Scientists have shown that these TSH receptor antibodies also activate retro-orbital fibroblasts and retro-orbital adipogenesis in eye socket tissue. Clinically, it has been observed that patients with high concentrations of stimulating thyroid-stimulating hormone receptor (TSHR) antibodies in their serum are more prone to moderate to severe TED, underscoring the integral role of TSHR-expressing cells in the pathogenesis of the disease.
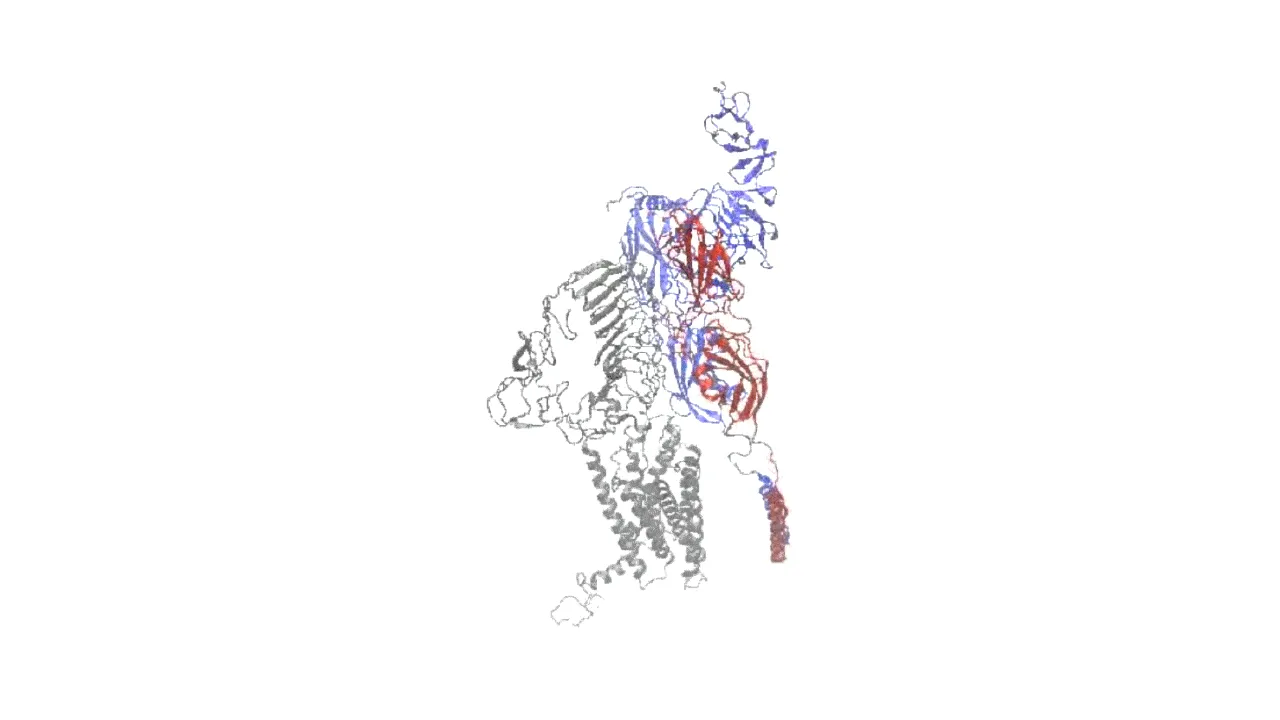
Figure shows the complex formed by the TSH receptor binding to the IGF-1 receptor.
About 40 percent of patients with Graves’ disease suffer from thyroid eye disease and its potentially severe side effects of proptosis, diplopia, pain, and inflammation. TED occurs when TSH receptor antibodies mistakenly target the TSH receptor on the surface of thyroid epithelial cells. Scientists have shown that these TSH receptor antibodies also activate retro-orbital fibroblasts and retro-orbital adipogenesis in eye socket tissue. Clinically, it has been observed that patients with high concentrations of stimulating thyroid-stimulating hormone receptor (TSHR) antibodies in their serum are more prone to moderate to severe TED, underscoring the integral role of TSHR-expressing cells in the pathogenesis of the disease.
While the additive effects of TSH and IGF-1 receptors have been known for years, Tepezza provided additional insights into their affinity by showing how blocking IGF-1 with a monoclonal antibody helped reduce inflammation and prevent expansion of connective tissue near the eyes. The Mount Sinai team has uncovered further biological evidence that these blocked cells, no longer receptor-stimulated, simply die off, allowing the bulging eye to return to its normal orbital position.
Long-time investigators at Mount Sinai provided valuable insights into this work. They are Mihaly Mezei, PhD, Associate Professor of Pharmacological Sciences, whose lab is well known for modeling and understanding complex molecular systems such as proteins and nucleic acids; and Rauf Latif, PhD, Associate Professor of Medicine (Diabetes, Endocrinology and Bone Disease), and Pharmacological Sciences, who has focused his lab’s work on understanding the biology of the TSH receptor, including its regulation and signaling.
“We have shown through our modeling and experimental approaches that TSHR and IGF-1R have an intricate choreography of protein-to-protein interactions to form a stable complex,” explains Dr. Davies, who was honored at the 2025 International Thyroid Congress in Brazil with a “Legends” award for his lifetime work on thyroid disease. “We’re now building on those findings through further mutational analysis to determine the responsible TSHR residues that may serve as important new therapeutic targets, and to evaluate the critical role of receptor signaling.”
Featured

Terry F. Davies, MD
Florence and Theodore Baumritter Professor of Medicine (Endocrinology, Diabetes and Bone Disease)

Rauf Latif, PhD
Associate Professor of Medicine (Diabetes, Endocrinology and Bone Disease), and Pharmacological Sciences

Mihaly Mezei. PhD
Associate Professor of Pharmacological Sciences