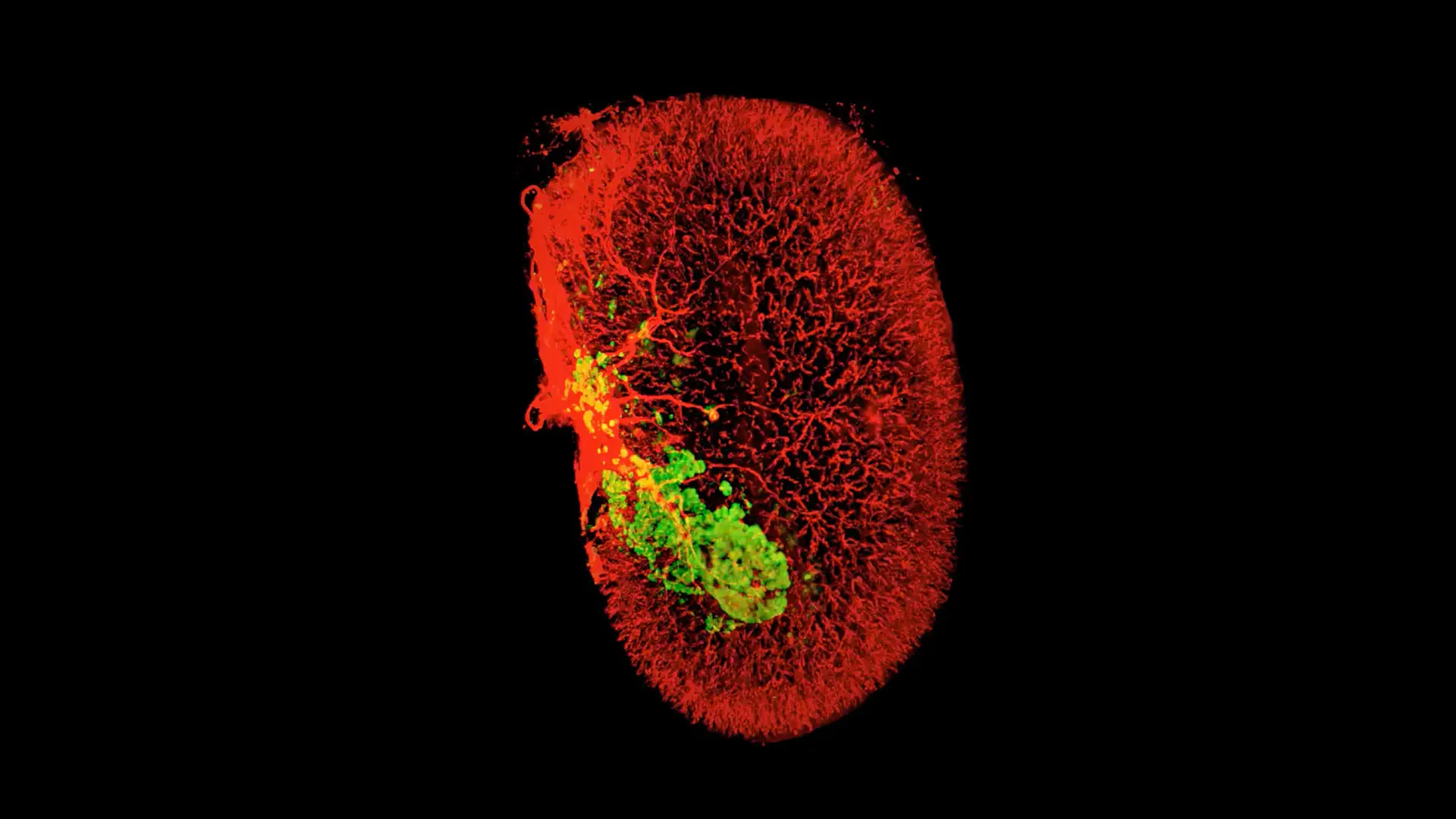
Mount Sinai researchers have found that a two-drug combination boosts regeneration of insulin-producing pancreatic beta cells without major adverse effects, a positive step toward a potential new way to treat diabetes.
The new work is the latest from a team led by Andrew E. Stewart, MD, Professor of Medicine (Endocrinology, Diabetes, and Bone Disease) and Director of the Diabetes, Obesity and Metabolism Institute (DOMI) at the Icahn School of Medicine at Mount Sinai. Since 2015, the team has been studying a natural plant ingredient it discovered called harmine, a small-molecule inhibitor of an enzyme called dual-specificity tyrosine-regulated kinase 1A (DYRK1A). When used in combination with glucagon-like peptide 1 receptor agonist (GLP-1RA), the two drugs promote replication of the beta cells that are nearly absent in type 1 diabetes and insufficient in type 2 diabetes.
“It’s always been scientific dogma that beta cells are terminally differentiated and can never replicate again. But we identified a small molecule called harmine, found naturally in a variety of plants, that actually can make human beta cells regenerate,” Dr. Stewart says.

Diabetic mice transplanted with human islets and treated with the harmine and exendin-4 combination displayed a rapid, considerable, and sustained normalization of blood glucose concentrations that was maintained for the entire 3 months of treatment and that was superior to the vehicle and single-treatment groups.
In July 2024, the team published a paper in Science Translational Medicine, showing that harmine plus the GLP-1RA exenatide increased the mass and function of human beta cells transplanted into both diabetic and nondiabetic mice. The effects were sustained for at least one month after the drugs were stopped.
“When we made the mice diabetic with streptozotocin, since they had none of their own beta cells, their blood sugar levels were around 300 or 400 [mg/dL]. When we treated them with harmine plus exenatide, their blood sugar normalized rapidly within a week and stayed completely normal for three months,” Dr. Stewart says.
When the researchers sacrificed the mice, they found that their human pancreatic beta cell mass had tripled with harmine alone, and increased sevenfold with the addition of exenatide. This is enough to fully replenish the lost beta cells in type 1 diabetes and type 2 diabetes.
A second paper, published in September 2024 in the Journal of Psychopharmacology, explores potential safety concerns. Harmine is a component of the hallucinogenic brew known as ayahuasca that humans have ingested for centuries as part of traditional religious ceremonies in parts of South America and increasingly as a mind-altering tea elsewhere, including the United States.
Ayahuasca has potent psychoactive, hallucinogenic effects that would prevent its use in people with diabetes. But ayahuasca contains many known and unknown components. So the Mount Sinai team wanted to know if harmine itself is hallucinogenic or whether the psychoactivity was due to other components in ayahuasca. In the phase 1, open-label trial in 25 healthy adults randomized to different doses of pure, pharmaceutical-grade harmine, they found that subjects did not experience hallucinations or other psychoactive effects. At higher doses, some subjects did experience mild to moderate and dose-dependent gastrointestinal side effects (nausea, vomiting).
“It quickly became clear that doses up to 2.7 mg/kg were well tolerated. Now we know how much healthy people can safely tolerate. And, we know from our animal studies that this is still in the range that can treat diabetes,” Dr. Stewart says.
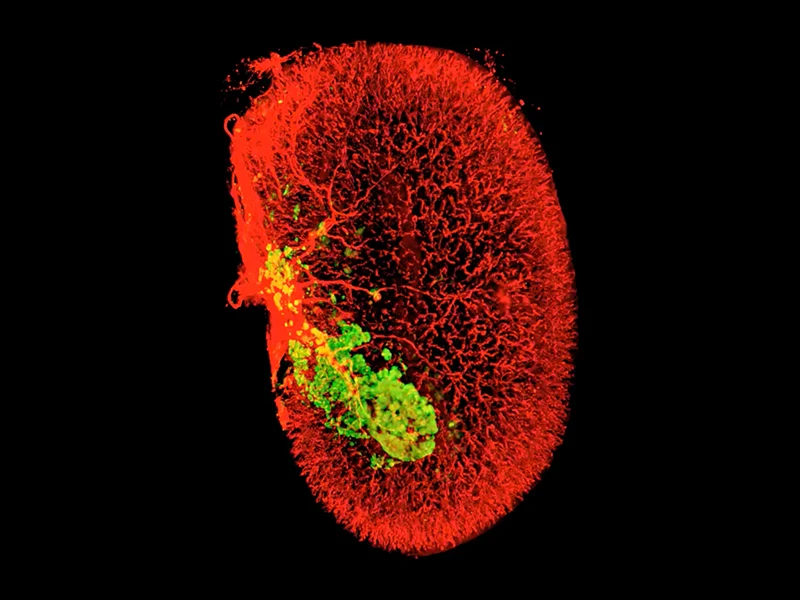
A 3D image of beta cells (green) in one human islet graft (smooth muscle actin, red) harvested from an untreated mouse 2 weeks after transplantation
“As compared to other strategies to replace beta cells, such as a pancreas transplant, islet transplant, or stem cell therapies, taking a harmine pill every day, alone or with a GLP-1RA such as Ozempic, would be simple and cost effective.”
Andrew F. Stewart, MD
Many challenges remain. Because type 1 diabetes is an autoimmune disease, other immunomodulatory agents will be required. One possibility might be teplizumab, a monoclonal antibody recently approved in the United States to delay progression of beta cell destruction in people identified with early stage (preclinical) type 1 diabetes.
“As compared to other strategies to replace beta cells, such as a pancreas transplant, islet transplant, or stem cell therapies, taking a harmine pill every day, alone or with a GLP-1RA such as Ozempic, would be simple and cost effective,” Dr. Stewart says.
And then there is the issue of bringing these discoveries to people with diabetes. Because harmine is a natural product, it can’t be patented. “If you can’t patent it, no drug company is going to make it because clinical trials are expensive,” he notes.
So, going forward, the team has turned its efforts toward other new small molecule compounds they have designed and synthesized, along with computer modeling. “Some of these are as good as harmine, and several are substantially better. Mount Sinai has patented these, and we’re now working to move them along via Mount Sinai’s Innovation Partners Program,” Dr. Stewart says.
The project is a Mount Sinai team effort with critical input from Robert DeVita, PhD, and Kunal Kumar, PhD, in the Drug Discovery Institute; Peng Wang, PhD, Carolina Rosselot, PhD, and Sarah A. Stanley, MBBCh, PhD, in DOMI; Jessica Ables, MD, PhD, and James Murrough, MD, PhD, in the Department of Psychiatry; and Adolfo Garcia-Ocaña, PhD, at the City of Hope.
The work is supported from multiple grants from the National Institutes of Health and also a recent $1 million grant from Breakthrough T1D (formerly JDRF).
Featured

Andrew F. Stewart, MD
Professor of Medicine (Endocrinology, Diabetes and Bone Disease) and Director of the Diabetes, Obesity and Metabolism Institute

Sarah Stanley, MBBCh, PhD
Associate Professor of Medicine (Endocrinology, Diabetes and Bone Disease)