The Diabetes, Obesity and Metabolism Institute (DOMI) at the Icahn School of Medicine at Mount Sinai made further outstanding progress in 2024 in the study of diabetes, obesity, and metabolic syndromes under the leadership of the Institute Director, Andrew F. Stewart, MD.
Dr. Stewart’s team furthered its pioneering research, demonstrating that a two-drug combination—glucagon-like peptide 1 receptor agonist (GLP-1RA) and harmine—enables the regeneration of insulin-producing pancreatic beta cells for people with diabetes. In another significant publication, Esra Karakose, PhD, advanced the understanding of the mechanisms of action for DYRK1A inhibitors and GLP-1RAs.
In a prestigious honor, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Catalyst Award program in 2024 awarded a $4 million, five-year grant to Prashant Rajbhandari, PhD, Assistant Professor of Medicine (Endocrinology, Diabetes and Bone Disease), for his study of hormones that he discovered, called “mammokines,” and their influence on the body’s metabolic health. And Dr. Rajbandari advanced the understanding of adipogenesis in a significant publication in Nature Communications. Dirk Homann, MD, and his team obtained funding from the National Institutes of Health, Breakthrough T1D, and the Helmsley Charitable Trust to extend research that aims to predict the development and progression of type 1 diabetes. And a team led by Donald Scott, PhD, and Liora Katz, PhD, furthered the understanding of the Carbohydrate-Response Element-Binding Protein (ChREBP) in potentially preserving beta cell mass.
In their research, DOMI researchers often collaborate closely with physician-scientists from departments throughout the Mount Sinai Health System, including endocrinology, surgery, genetics and genomics, and pharmacology.
“Over the years, we have recruited people internally and externally who are extremely driven, smart, and complementary to the focus of DOMI’s research mission,” Dr. Stewart says. “I am immensely proud of our DOMI teams as we work to find cures and to develop better therapeutic and prevention strategies for those who suffer from diabetes, obesity, and metabolic syndromes.”
Below are summaries of notable work in 2024 by Dr. Scott, Dr. Katz, Dr. Homann, Dr. Karakose, and Dr. Rajbhandari.

Donald Scott, PhD, and Liora Katz, PhD

Illustrating the mechanism by which small molecule stabilizers of the ChREBPα/14-3-3 protein-protein interaction (PPI) protect insulin-secreting beta cells from glucolipotoxicity. The optimized "molecular glue" compounds retain ChREBPα in the cytoplasm, preventing its transcriptional activity and subsequent beta cell dedifferentiation and death. This approach highlights a novel therapeutic strategy for maintaining beta cell identity and function in the context of type 2 diabetes.
Abstract of Study: “Molecular glues of the regulatory ChREBP/14-3-3 complex protect beta cells from glucolipotoxicity.” Biorxiv, November 2024
Preservation of functional beta-cell mass is a therapeutic goal for diabetes. The carbohydrate response element binding protein (ChREBP) is a glucose-responsive transcription factor (TF) with two major splice isoforms (α and β). In chronic hyperglycemia and glucolipotoxicity, ChREBPα-mediated ChREBPβ expression surges, leading to insulin-secreting beta-cell dedifferentiation and death. 14-3-3 binding to ChREBPα results in cytoplasmic retention and suppression of transcriptional activity. Thus, small molecule-mediated stabilization of this protein-protein interaction may be of therapeutic value. Here, we show that structure-based optimizations of a “molecular glue” compound led to potent ChREBPα/14-3-3 PPI stabilizers with cellular activity. In primary human beta cells, the most active compound retained ChREBPα in the cytoplasm, and efficiently protected beta cells from glucolipotoxicity while maintaining beta-cell identity. Thus, this study may not only provide the basis for the development of a unique class of compounds for the treatment of type 2 diabetes but also showcases an alternative “molecular glue” approach for achieving small molecule control of notoriously difficult-to-target TFs.
The Takeaway: Donald Scott, PhD, Professor of Medicine (Endocrinology, Diabetes and Bone Disease), and Liora Katz, PhD, Associate Professor of Medicine (Endocrinology, Diabetes and Bone Disease)
Diabetes results from insufficient functional beta cell mass. When blood glucose levels rise, such as in response to a high-fat diet, beta cells respond by producing and releasing more insulin to manage blood glucose levels. Prolonged hyperglycemia, which may occur due to insulin resistance, hinders the ability of beta cells to produce and secrete insulin. This leads to a vicious cycle of rising glucose levels and declining beta cell activity, eventually leading to the death or dedifferentiation of beta cells, a phenomenon known as glucose toxicity or “glucolipotoxicity,” leading to diabetes. Thus, preservation and regeneration of functional beta cell mass are important therapeutic goals for diabetes research. We discovered a molecular mechanism involving ChREBP, a glucose-sensing transcription factor with two major isoforms, that is involved in glucolipotoxicity. Transcription factors turn genes on and off. We found that ChREBPα activates a hyperactive isoform of this protein, ChREBPβ, in a feed-forward manner, which is then overproduced by prolonged hyperglycemia leading to glucolipotoxicity, dedifferentiation, and beta cell death. Thus, any therapy that prevents the destructive overexpression of ChREBPb should protect beta cells from glucolipotoxicity.
In collaboration with Luc Brunsveld, PhD, and Christian Ottmann, PhD, from the Eindhoven University of Technology; and Markus Kaiser, PhD, from the University of Duisburg-Essen, we have used a novel approach to sequester ChREBPα in the cytoplasm of beta cells, thus preventing the deleterious upregulation of ChREBPb and completely protecting beta cells from glucolipotoxicity. The European group used structure-based optimization to generate potent new “molecular glues,” compounds that increase the protein-protein interaction between 14-3-3, a protein that acts as a cytoplasmic anchor, and ChREBPα. This increased interaction keeps ChREBPα in the cytoplasm and prevents the production of the hyperactive ChREBPb isoform. We found three active compounds—potential future diabetes drugs—that safely enter beta cells, have no effect on beta cell function, and prevent nuclear localization of ChREBPα and the upregulation of ChREBPb, thereby completely protecting rodent and human beta cells from cell death and dedifferentiation from glucolipotoxicity. In studies now underway, we will test these compounds in diabetic mice to see if they protect beta cells from glucolipotoxicity. Since ChREBPα and ChREBPb are expressed in many metabolically active tissues that are impacted by diabetes, we are excited to test the effects of our molecular glues not only in beta cells, but also in liver and kidney cells. In summary, our findings create an opportunity to develop therapeutic agents that inhibit ChREBPβ production, and thus preserve beta cell mass, and may protect other tissues from the harmful effects of diabetic ChREBPb overexpression, as well.

From left, Andrew F. Stewart, MD, Esra Karakose, PhD, and Peng Wang, PhD
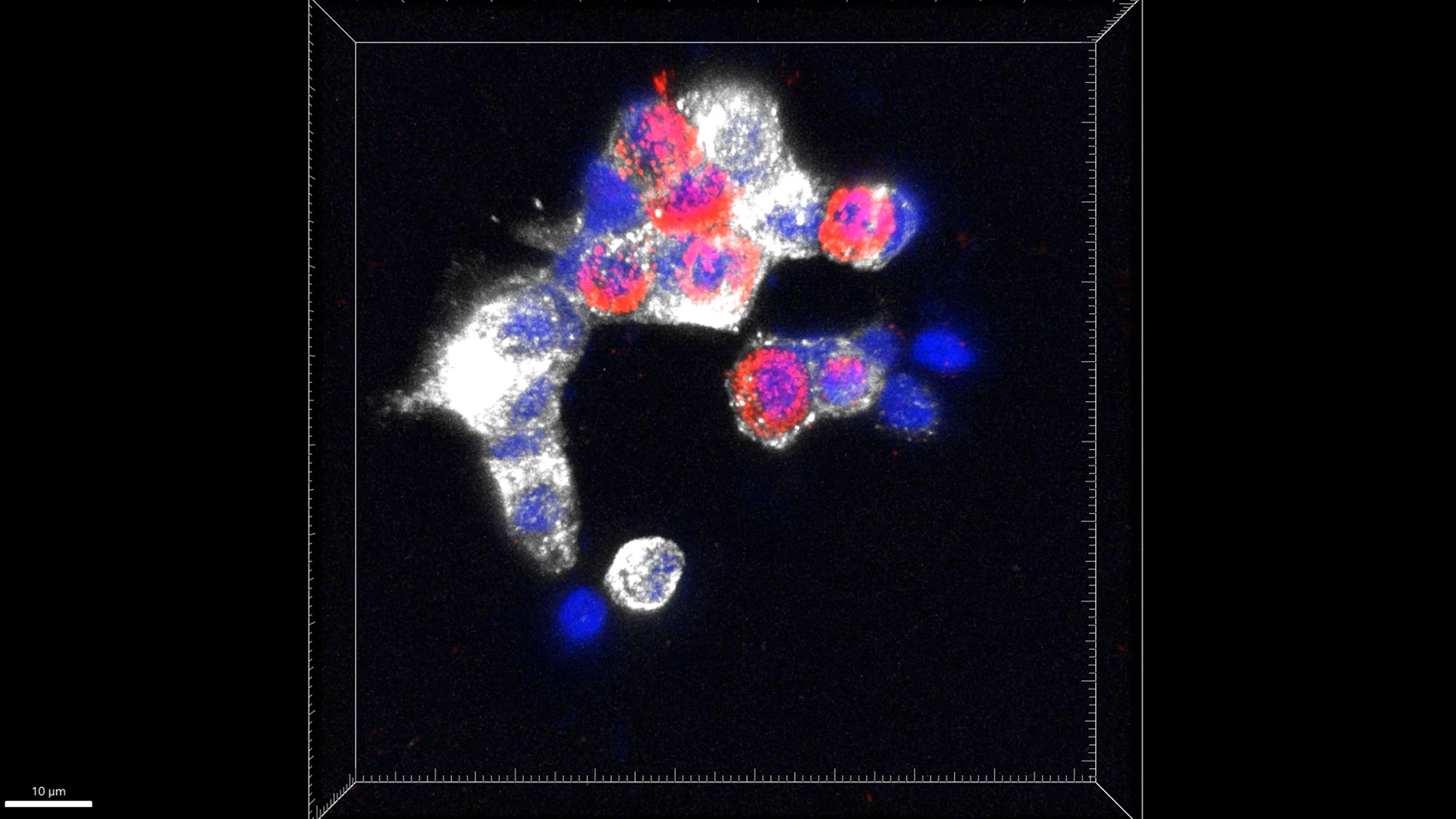
Depiction of clear colocalization of C-peptide and glucagon in human islets treated with harmine plus GLP-1.
Abstract of Study: Cycling alpha cells in regenerative drug-treated human pancreatic islets may serve as key beta cell progenitors, Cell Reports Medicine, December 2024
Diabetes results from an inadequate number of insulin-producing human beta cells. There is currently no clinically available effective means to restore beta cell mass in millions of people with diabetes. Although the DYRK1A inhibitors—either alone or in combination with GLP-1 receptor agonists (GLP-1) or transforming growth factor-b (TGF-b) superfamily inhibitors (LY)—induce beta cell replication and increase beta cell mass, the precise mechanisms of action remain elusive. Here we perform single-cell RNA sequencing on human pancreatic islets treated with a DYRK1A inhibitor, either alone or with GLP-1 or LY. We identify cycling alpha cells as the most responsive cells to DYRK1A inhibition. Lineage trajectory analyses suggest that cycling alpha cells may serve as precursor cells that transdifferentiate into beta cells. Collectively, in addition to enhancing expression of beta cell phenotypic genes in beta cells, our findings suggest that regenerative drugs may be targeting cycling alpha cells in human islets.
The Takeaway: Esra Karakose, PhD, Assistant Professor of Medicine (Endocrinology, Diabetes and Bone Disease)
Diabetes affects more than 500 million people worldwide. Since all diabetes ultimately results from insufficient numbers of insulin-producing beta cells, people with diabetes would benefit immensely from affordable beta cell regenerative drugs that provide a scalable approach to beta cell regeneration. The most advanced beta cell regenerative therapies include DYRK1A inhibitors, exemplified by harmine given alone, or with GLP-1 receptor agonists (GLP-1RAs). These produce dramatic (300-700 percent) increases in human beta cell mass over three months, enhance beta cell function, and reverse diabetes.
However, the mechanisms of action for DYRK1A inhibitors and GLP-1RAs are currently incompletely understood. In this publication, we demonstrate that human pancreatic islets contain a population of “cycling alpha cells” that serve as progenitor reservoir for new beta cells following DYRK1A inhibitor treatment. This strongly suggests that beta cell regenerative therapies that involve DYRK1A inhibitors may induce lineage conversion in human pancreatic islets. Since alpha cells are abundant in people with type 1 and type 2 diabetes, they may be able to serve as a reservoir for new beta cells in both common types of diabetes. In addition, since the effects of harmine and GLP1 do not seem to affect cells other than beta cells and alpha cells, our results suggest that beta cell targeting for DYRK1A inhibitors may no longer be needed.
Collectively, these results shed light on the mechanisms of action of DYRK1A inhibitors and GLP-1RAs and pave the way for future studies to better understand the lineage dynamics in human islets with beta cell regenerative drug treatment. To this end, we are initiating longitudinal studies in which we will study the effect of beta cell regenerative drugs on human islets at various time points. Further, we are also investigating lineage dynamics in an in vivo system where human islets are transplanted into immunodeficient mice that are treated with control or regenerative drugs.

From left: Chung Hwan Cho, PhD, Ramazan Yildez, PhD, Prashant Rajbhandari, PhD, James McMullen, PhD, David (Young Uk) Jang, MS, and Sanil Patel, MS.

A working model of PATZ1 role in adipogenesis and adipocyte maintenance by activating CEBPb-PPARg-CEBPa circuitry.
Abstract of Study: Transcription factor PATZ1 promotes adipogenesis by controlling promoter regulatory loci of adipogenic factors, Nature Communications, October 2024
White adipose tissue (WAT) is essential for lipid storage and systemic energy homeostasis. Understanding adipocyte formation and stability is key to developing therapies for obesity and metabolic disorders. Through a high-throughput cDNA screen, we identified PATZ1, a POZ/BTB and AT-Hook Containing Zinc Finger 1 protein, as an important adipogenic transcription factor. PATZ1 is expressed in human and mouse adipocyte precursor cells (APCs) and adipocytes. In cellular models, PATZ1 promotes adipogenesis via protein-protein interactions and DNA binding. PATZ1 ablation in mouse adipocytes and APCs leads to a reduced APC pool, decreased fat mass, and hypertrophied adipocytes. ChIP-Seq and RNA-seq analyses show that PATZ1 supports adipogenesis by interacting with transcriptional machinery at the promoter regions of key early adipogenic factors. Mass-spec results show that PATZ1 associates with GTF2I, with GTF2I modulating PATZ1’s function during differentiation. These findings underscore PATZ1’s regulatory role in adipocyte differentiation and adiposity, offering insights into adipose tissue development.
The Takeaway: Prashant Rajbhandari, PhD, Assistant Professor of Medicine (Endocrinology, Diabetes and Bone Disease)
Adipogenesis—the finely tuned process of developing and accumulating fat cells—is governed by a cascade of transcriptional events, yet many key regulatory factors remain unidentified. Our study positions PATZ1 as a novel, essential transcriptional regulator of white adipocyte development. While PATZ1 was previously studied in stem cell biology and cancer, its role in adipose tissue biology was unexplored. We show that PATZ1 promotes adipogenesis through both direct transcriptional regulation of early adipogenic genes and protein–protein interactions, including with GTF2I, a general transcription factor that modulates its activity. Interestingly, PATZ1 deletion reduces the APC pool and fat mass but paradoxically results in adipocyte hypertrophy—suggesting that PATZ1 not only drives adipocyte formation but may also constrain adipocyte expansion. This duality highlights the complexity of transcriptional control in adipose tissue development and homeostasis. Our findings open new questions about how PATZ1 coordinates with other chromatin remodelers and transcriptional networks during early lineage commitment. Given the increasing interest in targeting adipocyte plasticity for metabolic disease therapy, PATZ1 could represent a new molecular handle to manipulate adipogenesis. Future studies will explore how PATZ1 function is integrated with nutrient or hormonal cues, and whether its regulatory axis can be therapeutically modulated to reverse adipose tissue dysfunction in obesity and lipodystrophy.
KQ1 serum study: Predicting type 1 diabetes development and progression
A team led by Dirk Homann, MD, and Co-Principal Investigator Cate Speake, PhD, of the Benaroya Institute in Seattle, in 2024 obtained major funding from the National Institute of Diabetes and Digestive and Kidney Disorders, Breakthrough T1D, and the Helmsley Charitable Trust to extend the Key Question 1 (KQ1) study, which aims to predict the development and progression of type 1 diabetes (T1D).
With a disease prevalence exceeding 0.3 percent in the United States and an incidence that has been increasing in recent years, T1D poses a considerable challenge to afflicted individuals and their families, to the development of effective prevention and treatment regimens, and to public health initiatives at large. While the precise interactions between inherited susceptibilities and environmental factors remain to be fully elucidated, T1D development is mediated by a complex interplay of aberrant immune responses that damage and destroy insulin-secreting pancreatic beta cells, leading to elevated blood glucose levels as well as serious disturbances of protein, fat, and carbohydrate metabolism. To date, no cure is available, and despite notable advances in disease management (insulin pumps, continuous glucose monitoring), overall glucose control has not fundamentally improved, and long-term complications such as kidney failure, heart attack, and stroke remain frequent.
The natural history of T1D unfolds in a series of events that progress at variable rates toward onset of clinical disease. Yet the precise correlates and determinants of T1D pathogenesis remain incompletely defined. The identification and validation of novel biomarkers is critical to promote better stratification of T1D risk and progression, can illuminate pathways of disease pathogenesis and heterogeneity, and will inform the development of interventional strategies to delay or prevent disease. To precipitate the identification of robust and improved biomarkers, the TrialNet consortium designed the KQ1 study, a nested case/control study of 178 at-risk (multiple auto-antibody positive) individuals who over a period of three years either did or did not progress to clinical disease. Earlier work aimed at correlating the evolution of phenotypic and transcriptomic immune cell profiles (conducted by Adeeb Rahman, PhD, and Dr. Homann at Mount Sinai; and Eoin McKinney, MD, PhD, at the University of Cambridge) with incipient T1D onset has been completed, and a manuscript has been submitted. The results indicate that T1D progression is associated with an increased abundance of effector precursor, effector, and senescent CD8+T cell subsets.
With the new funding, the team will extend the KQ1 study to a comprehensive interrogation of serum samples obtained from the same KQ1 donors and original sampling time points.
The Takeaway: Dirk Homann, MD, Professor of Medicine (Endocrinology, Diabetes and Bone Disease)
The KQ1 serum study has three aims: to identify and validate serum biomarkers for T1D risk, development, and/or progression; to establish a comprehensive data resource for reference and orientation, hypothesis generation, and improved interventional study design; and to execute broadly integrated data analyses in particular with the already available transcriptional and phenotypic immune cell data. To this end, we have identified and prioritized suitable targets and analytical approaches, robust technology platforms, and multidisciplinary investigator teams with track records to perform high-throughput analyses.
Our analyses include expansive measurements of serum proteins and metabolome-exposome profiles; antibody responses against autoantigens, viral components and commensal bacteria; blood cell responder transcriptomics; microRNA signatures; and exocrine pancreatic factors; as well as islet hormones and prohormones. Integrative analytics will be leveraged to define novel serum signatures for T1D risk stratification, improve prediction of disease progression rates, and elucidate aspects of T1D pathogenesis that may accelerate the development of tailored diagnostic, prophylactic, and therapeutic modalities. The KQ1 serum study is arguably the most comprehensive T1D serum screening effort conceived to date, and has unique potential to build a robust framework for future integration of serum biomarkers in the improved management of T1D.