Carol J. Levy, MD, Director of the Mount Sinai Diabetes Center, and her team made significant progress in 2024, working to broaden the reach of diabetes technology to more people who could benefit from it, including pregnant women with type 1 diabetes and people with type 2 diabetes who require multiple daily doses of insulin.
The team has expanded research on the use of automated insulin delivery (AID) systems among the subset of people with type 2 diabetes who use insulin. They are also investigating the use of faster-acting insulins for some patient groups, and Dr. Levy has continued her pioneering work in a field close to her heart, the use of diabetes technology during pregnancy.
“We’ve been reaching out to people with diabetes who have been missed in the past when it comes to technological advances,” says Dr. Levy, Professor of Medicine (Endocrinology, Diabetes and Bone Disease) and Associate Chief for Endocrine Clinical Research, Icahn School of Medicine at Mount Sinai.
Dr. Levy is the Mount Sinai lead investigator for a multicenter, 13-week trial of AID technology in people with type 2 diabetes. “Results of our previous pilot study were robust enough that it was deemed appropriate to proceed with a larger pivotal trial to submit for approval from the U.S. Food and Drug Administration,” she says.
That pivotal study was sponsored by Tandem Diabetes Care, Inc., maker of the t-slim X2 insulin pump with Control-IQ technology 1.5. This system is well known to Dr. Levy’s team since they participated in many earlier trials of this system in the academic setting. Used in combination with a continuous glucose (CGM) sensor, it is one of several currently available AID systems that automatically deliver insulin based on blood glucose readings and in response to user inputs regarding food and exercise.
Currently, AID systems are only approved for use in people with type 1 diabetes. But the data show that they can benefit people with type 2 diabetes who require multiple daily insulin injections. “Our hope is that this will soon be a treatment option for patients with type 2 diabetes,” Dr. Levy says.

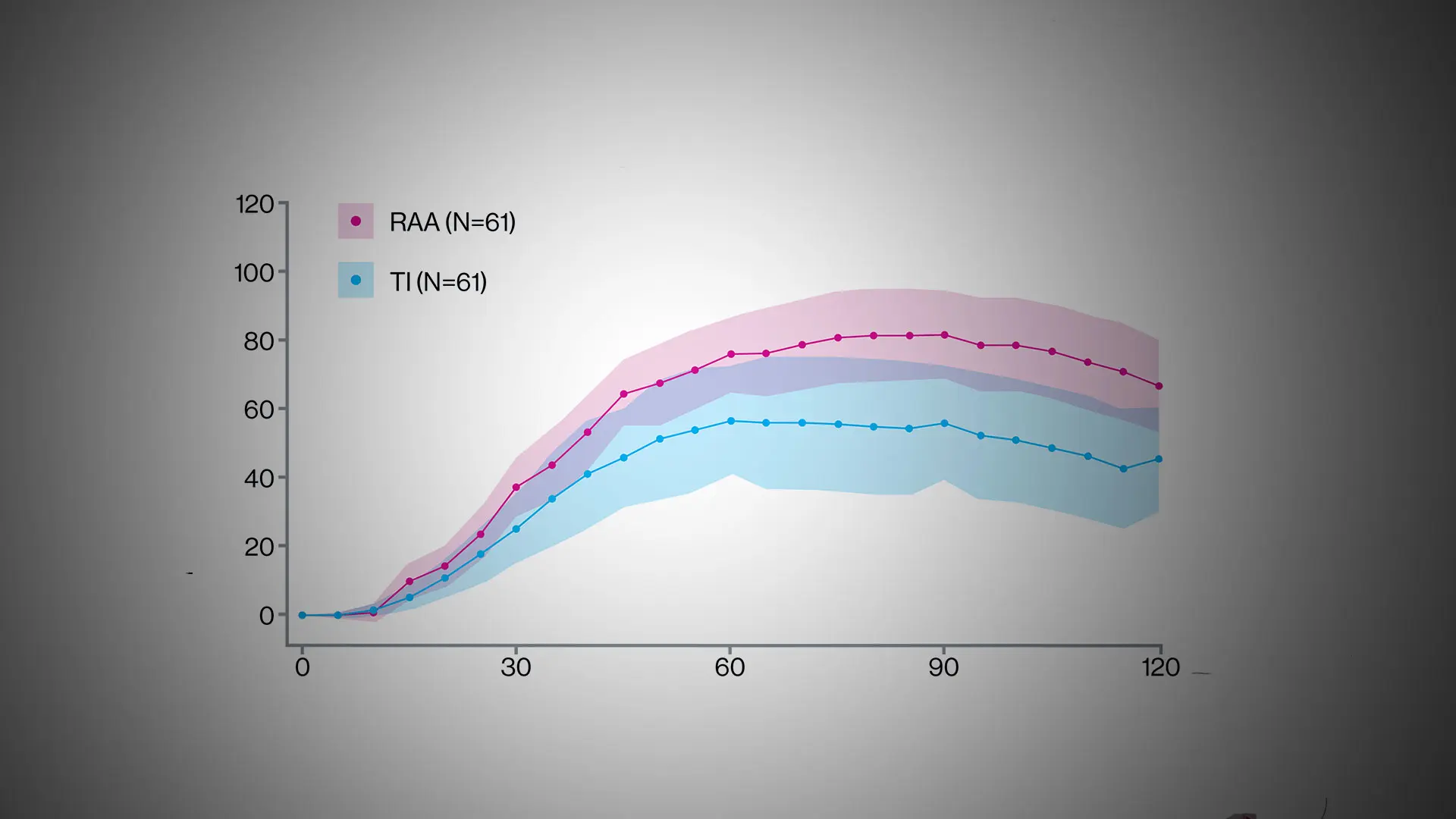
Graphical abstract of the September 2024 study in Diabetes Care.
“We’ve been reaching out to people with diabetes who have been missed in the past when it comes to technological advances.”
Carol J. Levy, MD
While AID system use has demonstrated the ability to improve glucose outcomes, many people with diabetes still struggle with meal dosing due to the slowness of current short-acting injectable insulin analogs. The result is that blood glucose levels often rise above high ranges after meals. So, in the past year, Dr. Levy and her team have published studies on use of faster-acting insulin analogs.
One study, published in September 2024 in Diabetes Technology & Therapeutics, showed that use of ultra-rapid lispro insulin in the Tandem AID system was safe for adults and children with type 1 diabetes, and that patients reported quality-of-life benefits. “The study demonstrated increased user satisfaction. Clinicians ask patients to wait 20-30 minutes after dosing insulin to eat their meals. This can be extremely challenging and requires a lot of planning. In a restaurant, it is nearly impossible for people to follow. Using an insulin that starts to work sooner reduces the time an individual needs to wait after dosing to eat. “While glucose levels remained similar for most participants, there is excitement about this option to reduce self-care burden,” Dr. Levy notes.
Another faster-acting meal dosing option requires no injecting or waiting after dosing to eat. Inhaled insulin (Afrezza) reduces post-meal glucose levels without the need for insulin injections. In two studies of adults with type 1 diabetes—with meal results published in September 2024 in Diabetes Care and overall outcomes published in December 2024 in the journal—Dr. Levy served as the lead investigator at Mount Sinai and presented the primary outcomes at the American Diabetes Association’s Scientific Sessions in June 2024.
Inhaled insulin acts immediately, but is not widely used. In part, that is because it is not incorporated into AID systems, which are increasingly seen as standard of care in type 1 diabetes. “In this randomized control trial, there was a clear subpopulation who had marked improvement in glucose control and greater satisfaction with inhaled insulin versus insulin pump use or standard multiple daily injection,” Dr. Levy explains.
She and other clinicians believe that inhaled insulin offers an important option for people who prefer or are not willing or able to wear an automated insulin delivery via an insulin pump, due to scar tissue that prevents use of AID systems. “Some people with longer duration diabetes run out of sites to deliver insulin. This provides an effective alternative.” There is another group who could potentially benefit from inhaled insulin: Women with gestational diabetes. “Think about women who are diagnosed with diabetes at 24 to 28 weeks of pregnancy. With this new diagnosis, many become overwhelmed. They are suddenly told to change their diet, test their blood sugars, and inject insulin four times a day. These future parents want to do the best for their baby, but it’s a lot for them to learn. Inhaled insulin could help ease the burden,” Dr. Levy says.
Dr. Levy is part of a five-site, investigator-initiated feasibility trial of inhaled insulin in gestational diabetes in early 2025, in collaboration with “other pregnancy-focused physicians who really believe that this has a place for these individuals.”
For pregnant women with type 1 diabetes, Dr. Levy in 2024 secured a commercial partner for a pregnancy-specific AID algorithm, after years of research and negotiations. A pregnancy-specific algorithm is necessary because current commercial AIDs do not bring glucose levels into the range required for pregnancy. “In 2023, in our feasibility study, we showed a system like this is feasible,” Dr. Levy says. “Now that we have a pump partner, we're going to proceed with larger studies. It’s incredibly exciting.”
Featured

Carol J. Levy, MD
Director of the Mount Sinai Diabetes Center, and Acting Chief and Professor of Medicine (Endocrinology, Diabetes and Bone Disease)

Grenye O'Malley, MD
Associate Professor of Medicine (Endocrinology, Diabetes and Bone Disease)