Mount Sinai researchers have made breakthrough discoveries about the thyroid-stimulating hormone (TSH) receptor that suggest new approaches to the treatment of autoimmune thyroid disease.
For decades, Terry F. Davies, MD, the Florence and Theodore Baumritter Professor of Medicine (Endocrinology, Diabetes and Bone Disease) at the Icahn School of Medicine at Mount Sinai, has studied the G-coupled protein TSH receptor, the autoantibody target in Graves’ disease and other autoimmune thyroid diseases.
“The current treatment of Graves’ disease hasn’t changed in 50 years. There are major disadvantages to the tablets that are used. They can cause bad side effects occasionally, they’re very slow to work, and there’s a 50 percent recurrence rate when you stop them. So, everyone would like to find a new treatment for Graves’ disease,” Dr. Davies says. “One of the obvious approaches would be to find something that blocks the antibody from binding to the TSH receptor. In order to do that, we need to know all about the TSH receptor.” Dr. Davies is a physician-scientist who practices at Mount Sinai Doctors in Manhattan and at the James J. Peters Department of Veterans Affairs Medical Center in the Bronx.
In 2022, Dr. Davies, along with Mount Sinai co-investigators Rauf Latif, PhD, and Mihaly Mezei, PhD, published in eLife the first complete model of the TSH receptor, derived via an artificial intelligence-based program called Alphafold2 combined with molecular dynamics stimulation. Dr. Latif is Associate Professor of Medicine (Endocrinology, Diabetes, and Bone Disease), and Dr. Mezei is Associate Professor of Pharmacological Sciences at Icahn Mount Sinai.
The team showed that the “hinge” or “linker” region that connects the cell membrane to the outer part of the receptor often changes its shape, but then becomes more stabilized after binding with TSH. That shape shifting “is why people always had trouble making a model of the TSH receptor in the past,” Dr. Davies says.
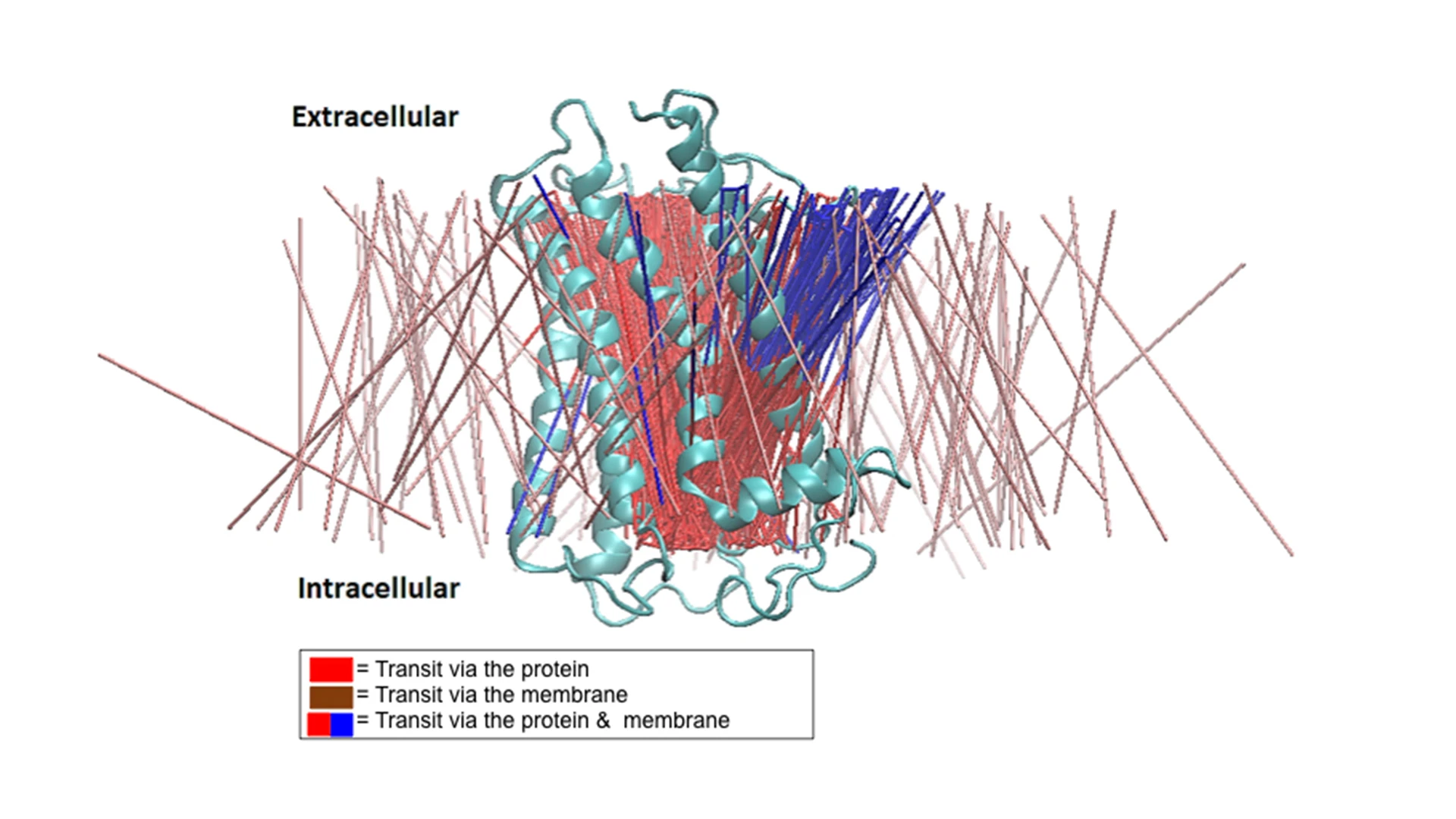
A lateral view of the transit lines extracted from the molecular dynamics simulation data representing 370 transits observed. The brown lines represent transit (diffusion) via the membrane, the red lines represent transit via the TSHR protein only, and the blue lines represent the path branching from the TSHR-TMD protein into the membrane, suggesting a bifurcated transit of the waters.

From left: Mihaly Mezei, PhD, Ping Ping Xiang, PhD, Terry F. Davies, MD, Syed Morshed, PhD, Maryam Mansoori, PhD, and Rauf Latif, PhD
In a study published in November 2023 in Endocrinology, the three investigators reported on experiments in the TSH receptor’s more stable transmembrane domain, located in the plasma membrane and consisting of lipids and water molecules. “Publication of the full-length receptor for the first time thanks to AI has now allowed us to concentrate more on the bricks that build it,” Dr. Davies says. “One of those bricks is the transmembrane section. That’s the bit that goes through the cell membrane to give the message to the cell to get busy.”
Using their previous experience in modeling and molecular dynamic simulations of the TSH receptor–transmembrane domain, “We found there was a passageway through the TSH receptor through which water could flow. This came as a surprise to all of us,” Dr. Davies says.
The transport time observed in the simulations via the TSH receptor was slower than via the enclosing cell membrane itself, however, significantly more water traversed via the TSH receptor than via the cell membrane. In addition, this effect more than doubled with the addition of TSH itself.
Then, by measuring thyroid cell volumes after blockade of major thyroid cell water transporters called aquaporins, the team found that TSH showed a dose-dependent ability to influence water transport. Similar effects were observed with stimulating TSH receptor autoantibodies seen in Graves’ disease.
The team also mapped the water channel pathway, showing that water movement may influence activation of the receptor. “This research suggests that TSH may influence water movement in all cells where the TSH receptor is expressed, which includes many non-thyroid tissues, including the brain, the bones, fibroblasts, and adipocytes,” Dr. Davies notes.
The water channel findings also call into question the long-held belief that the fluid retention and effusion formation that often occur with high TSH levels in hypothyroidism are due to a lack of thyroid hormone. In fact, there is no evidence supporting that assumption. Rather, “Our description of TSH receptor water channels opens an entirely new way of looking at fluid balance in hypothyroidism,” the investigators write in the paper.
These findings have implications for treating autoimmune thyroid disease and potentially other autoimmune conditions in which the immune target is a G-protein coupled receptor, Dr. Davies says.
“We think the water is very important for controlling the signaling of the receptor. If we want to inhibit the receptor in Graves’ disease, one possibility is inhibiting the water channel, because that should stop the receptor being so active. We’ve got drugs that activate the receptor, so we’re looking for a drug that might do the opposite.”
Featured

Terry F. Davies, MD
Florence and Theodore Baumritter Professor of Medicine (Endocrinology, Diabetes and Bone Disease)

Rauf Latif, PhD
Associate Professor of Medicine (Diabetes, Endocrinology and Bone Disease), and Pharmacological Sciences

Mihaly Mezei. PhD
Associate Professor of Pharmacological Sciences
