In ongoing, pioneering work, researchers at Mount Sinai have developed a new class of drugs known as small molecule DYRK1A (dual tyrosine-regulated kinase 1A) inhibitors, with the ability to induce adult human insulin-producing beta cells to proliferate and enhance their differentiation and function. The team made a crucial advance in 2022, describing the unique mechanism of this class of drugs in a paper published in the Journal of Clinical Investigation.
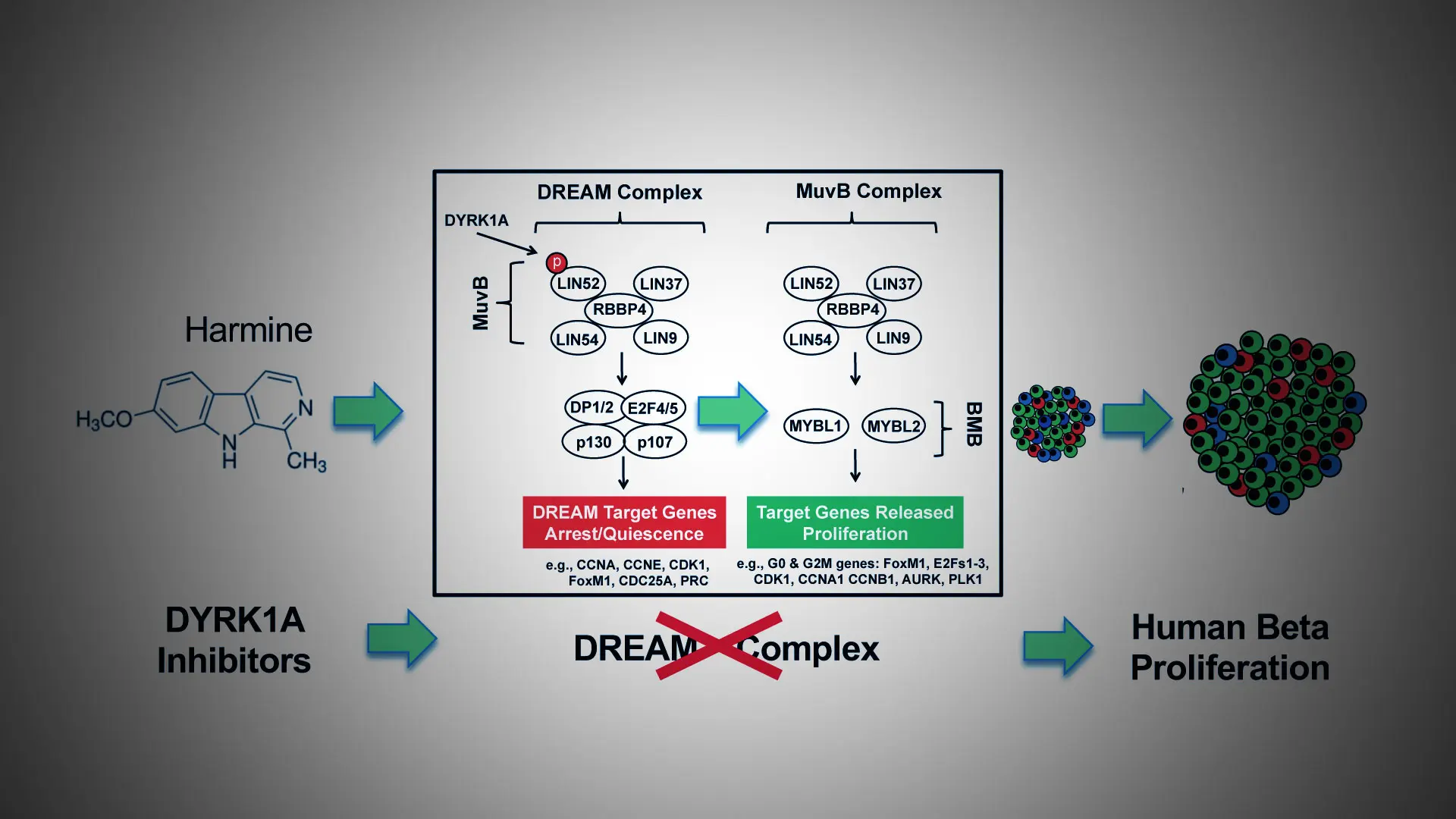
“We learned that a class of proteins called the DREAM complex acts as a central regulator of quiescence in human beta cells—in effect a natural set of ‘brakes’ on human beta cell proliferation,” says senior author Andrew F. Stewart, MD, Professor of Medicine (Endocrinology, Diabetes and Bone Disease) at the Icahn School of Medicine at Mount Sinai, and Director of the Diabetes, Obesity and Metabolism Institute. “The DYRK1A class inhibitors—exemplified by the inhibitor harmine—disrupt the DREAM complex by removing the brakes and allowing beta cells to regenerate and expand in number, thus reversing diabetes.”
Even higher rates of beta cell proliferation can be achieved by combining harmine with peptides agonists of the GLP1 receptor, or with more stable synthetic analogs such as exendin-4, which previous studies have shown to increase beta cell mass in immunodeficient mice transplanted with human islets by 700 percent over three months of treatment, significantly impacting diabetes.
“For the first time, we report that small molecule DYRK1A inhibitors convert the DREAM complex from its repressive configuration to a proliferative beta cell conformation,” says Peng Wang, PhD, Professor of Medicine (Endocrinology, Diabetes and Bone Disease) at Icahn Mount Sinai, and lead author of the study. “Our comprehensive model for control of the cell cycle in human beta cells alters a well-established paradigm in the field of diabetes, and provides a mechanism of action to explain how DYRK1A inhibitors induce beta cells to replicate.”

Team members, from left: Olivia Wood, BS; Lauryn Choleva, MD; Sharon Alterzon, PhD; Luca Lambertini, PhD; Andrew F. Stewart, MD; Peng Wang, PhD; Hongtao Liu, BS; and Liora Katz, PhD.
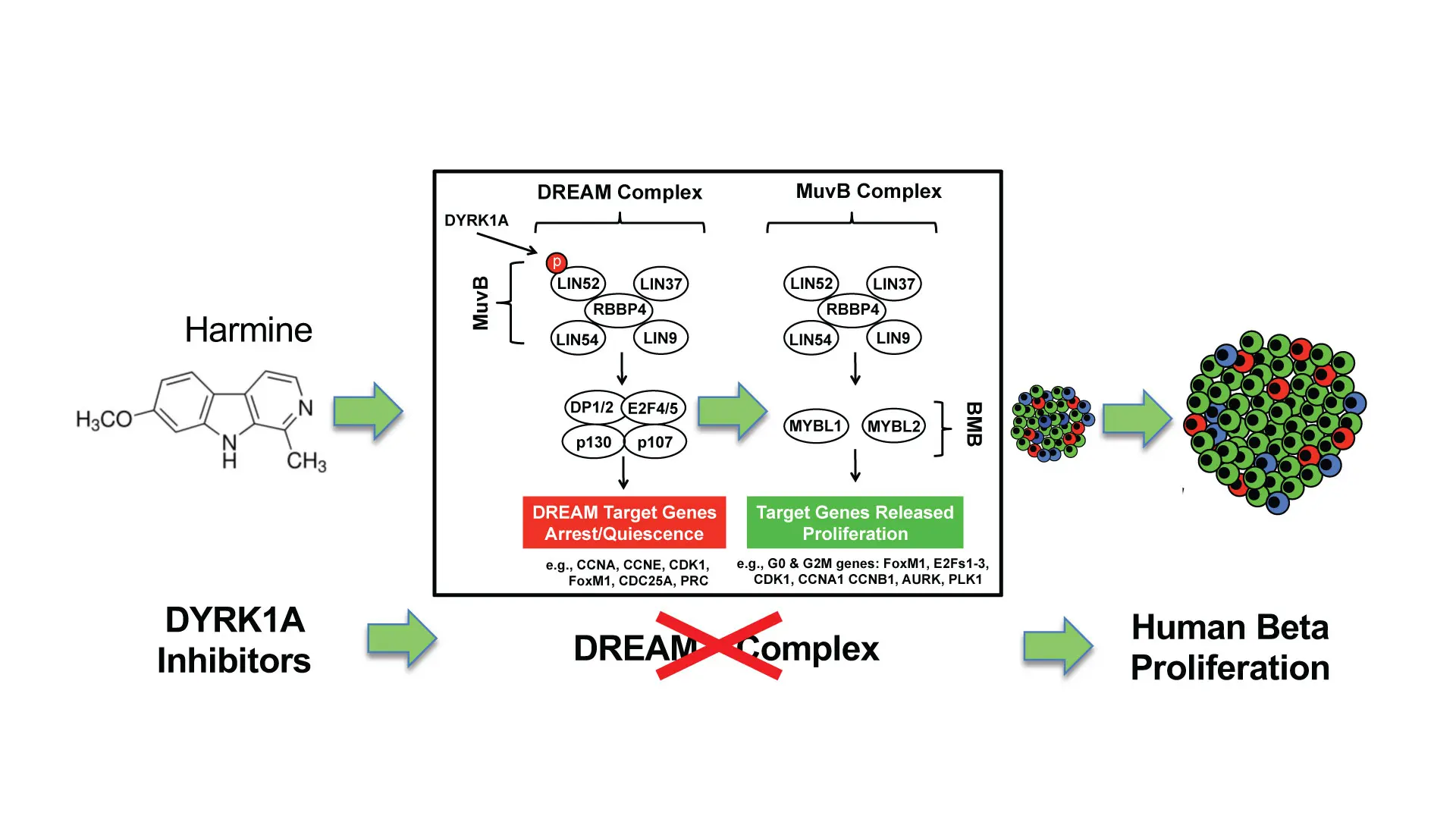
A graphical abstract of the study "Disrupting the DREAM complex enables proliferation of adult human pancreatic beta cells."
Regeneration of insulin-producing pancreatic beta cells has become the holy grail for researchers and clinicians as they search for alternative ways to treat a soaring worldwide population of 536 million people with type 1 and type 2 diabetes. Among the novel approaches are replacing missing beta cells by a whole pancreas transplant, or transplanting either isolated pancreatic islets from organ donors or beta cells derived from human stem cells. While each pathway has made considerable progress, none is scalable to the point it could help millions of people with diabetes due to cost and donor organ availability.
Researchers at Mount Sinai are well on their way to overcoming that hurdle. They are taking a pharmacologic approach to induce regeneration and redifferentiation of residual beta cells that remain in the pancreas of almost everyone with type 1 and type 2 diabetes.
The 2022 study is a vital part of a larger effort by Mount Sinai to bring DYRK1A inhibitors to patients with diabetes. Further impetus was provided by U.S. Food and Drug Administration approval of a phase 1, first-in-human trial of harmine, funded by the National Institute of Diabetes and Digestive and Kidney Disease at the National Institutes of Health. This recently launched study is providing participants with a single low dose of harmine, gradually escalating to higher doses in order to establish the maximum tolerable level of the inhibitor in humans. This information will be critical to planning longer-term studies in people with diabetes.
“Progress in the past has been limited by the lack of understanding by scientists of the biological mechanisms governing the repression vs. regenerative ability of beta cells,” notes Dr. Stewart, a leading authority for the past 40 years in diabetes research. “Our work is taking the field in an exciting new direction where, hopefully, the lifelong need by patients for daily injections or infusions of insulin will become outdated.”
Grand Rounds: Human Pancreatic Beta Cell Regeneration for Diabetes: A Journey From Impossible to Possible
Featured

Andrew F. Stewart, MD
Professor of Medicine (Endocrinology, Diabetes and Bone Disease) and Director of the Diabetes, Obesity and Metabolism Institute

Peng Wang, PhD
Professor of Medicine (Endocrinology, Diabetes and Bone Disease)