The Diabetes, Obesity and Metabolism Institute (DOMI) at the Icahn School of Medicine at Mount Sinai was created to develop better therapeutic and prevention strategies for those who suffer from diabetes, obesity, and metabolic syndromes. Under the leadership of Andrew F. Stewart, MD, Professor of Medicine (Endocrinology, Diabetes and Bone Disease), the Institute has expanded significantly in the last 10 years, with physician-scientists from departments throughout the Mount Sinai Health System—including Endocrinology, Surgery, Genetics and Genomics, and Pharmacology and Systems Therapeutics.
“There has been striking growth in faculty under Dr. Stewart’s leadership, rising from 3 in 2012 to 25 in 2022,” says Andrea Dunaif, MD, Chief of the Hilda and J. Lester Gabrilove Division of Endocrinology, Diabetes and Bone Disease, Icahn Mount Sinai. “And they're engaged in a variety of research areas related to the pathophysiology of both type 1 and type 2 diabetes.”
“It is hard to imagine a more exciting time in basic and translational biomedical research,” says Dr. Stewart. “The combination in 2022 of advanced technologies, DNA sequencing, proteomics, bioinformatics, robotics, artificial intelligence, and drug discovery, to name a few, allows us to make novel discoveries on an almost daily basis. Mount Sinai is rich in these capabilities: this environment is the primary driver of our advances in human beta cell regeneration for diabetes.”
Significant efforts in 2021 at DOMI include Dr. Stewart’s innovative research into beta cell regeneration, and the following research led by the physicians and scientists Dirk Homann, MD, Professor of Medicine (Endocrinology, Diabetes and Bone Disease); Prashant Rajbhandari, PhD, Assistant Professor of Medicine (Endocrinology, Diabetes and Bone Disease); and Sarah Stanley, MBBch, PhD, Associate Professor of Medicine (Endocrinology, Diabetes and Bone Disease), and Neuroscience.
Homann Lab

Team members, from left: Sonia Jangra, PhD; Michael A. Schotsaert, PhD; Dirk Homann, MD; and Verena van der Heide, MD, PhD.
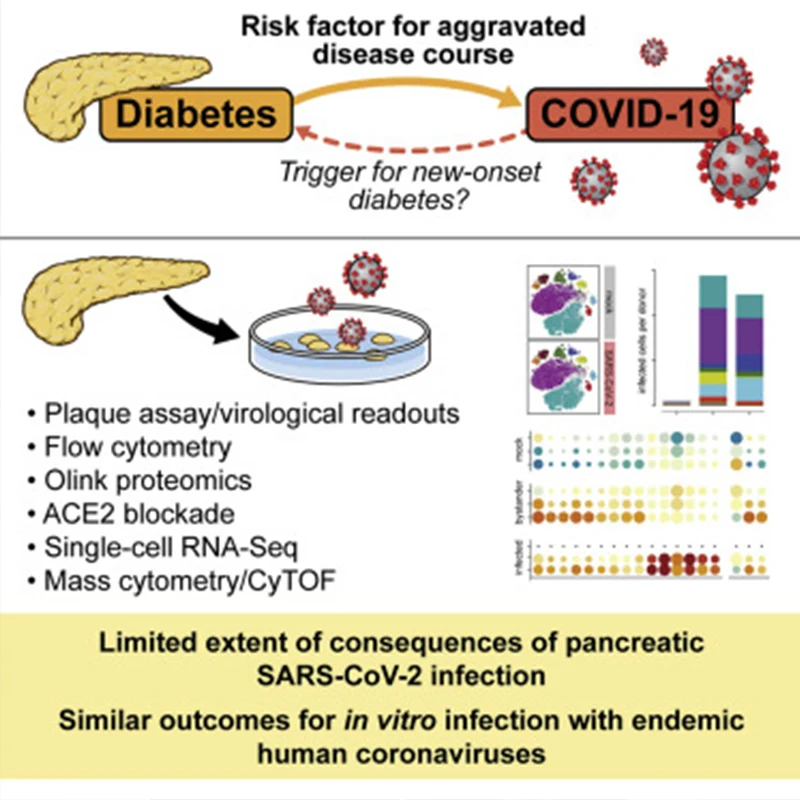
Publication:
"Limited Extent and Consequences of Pancreatic SARS-CoV-2 Infection" Cell Reports, February 2022
Summary:
Concerns that infection with SARS-CoV-2, the etiological agent of COVID-19, may cause new-onset diabetes persist amidst an evolving research landscape, and precise risk assessment is hampered by at times conflicting evidence. Here, leveraging comprehensive single-cell analyses of in vitro SARS-CoV2-infected human pancreatic islets, we demonstrate that productive infection is strictly dependent on the SARS-CoV-2 entry receptor ACE2 and targets practically all pancreatic cell types. Importantly, the infection remains highly circumscribed, largely non-cytopathic, and despite high viral burden in infected subsets, promotes only modest cellular perturbations and inflammatory responses. Similar experimental outcomes are also observed after islet infection with endemic coronaviruses. Thus, the limits of pancreatic SARS-CoV-2 infection, even under in vitro conditions of enhanced virus exposure, challenge the proposition that in vivo targeting of beta cells by SARS-CoV-2 precipitates new-onset diabetes. If restricted pancreatic damage and immunological alterations accrued by COVID-19 increase cumulative diabetes risk, however, remains to be evaluated.
The Takeaway, from Dirk Homann, MD:
When the pandemic hit in early 2020, we had the opportunity to leverage our familiarity with both infectious diseases and diabetes to test the hypothesis, prominently advanced by some of our colleagues in the field, that SARS-CoV-2 infection, the cause of COVID-19, may also trigger new-onset diabetes.
In a first step, we investigated expression patterns of the molecular machinery in the normal and diabetic human pancreas that may permit infection with SARS-CoV-2. The bottom line in a Cell Metabolism paper from December 2020 is that some pancreatic structures such as ducts and microvasculature can in principle accommodate a productive virus infection, but endocrine cells, including beta cells, for the most part cannot. Thus, direct targeting and incapacitation of beta cells by SARS-CoV-2 and promotion of new-onset diabetes would appear to be unlikely events.
We have since received NIH funding for this work, and in a second paper, published in Cell Reports in February 2022, we have employed the deliberate in vitro infection of human islets with SARS-CoV-2. While this scenario allows for infection of practically all pancreatic cell types, the overall extent and consequences of infection remain rather limited. We also observed similar experimental outcomes with endemic human coronaviruses not associated with enhanced diabetes risk. Altogether, this work emphasizes that results from such in vitro studies have to be interpreted with great caution, and any conclusions have to be tempered by observations made in complementary studies, most importantly the emerging epidemiological evidence.
Essentially, what we are saying is that there is a lot to worry about when it comes to COVID-19, including prolonged disturbances of glucose metabolism in “long-COVID” and perhaps even an enhanced cumulative risk for future diabetes development in at-risk populations. But new-onset diabetes as a direct consequence of SARS-CoV-2 infection is not one of those concerns.
Stanley Lab

Sarah A. Stanley, MBBCh, PhD
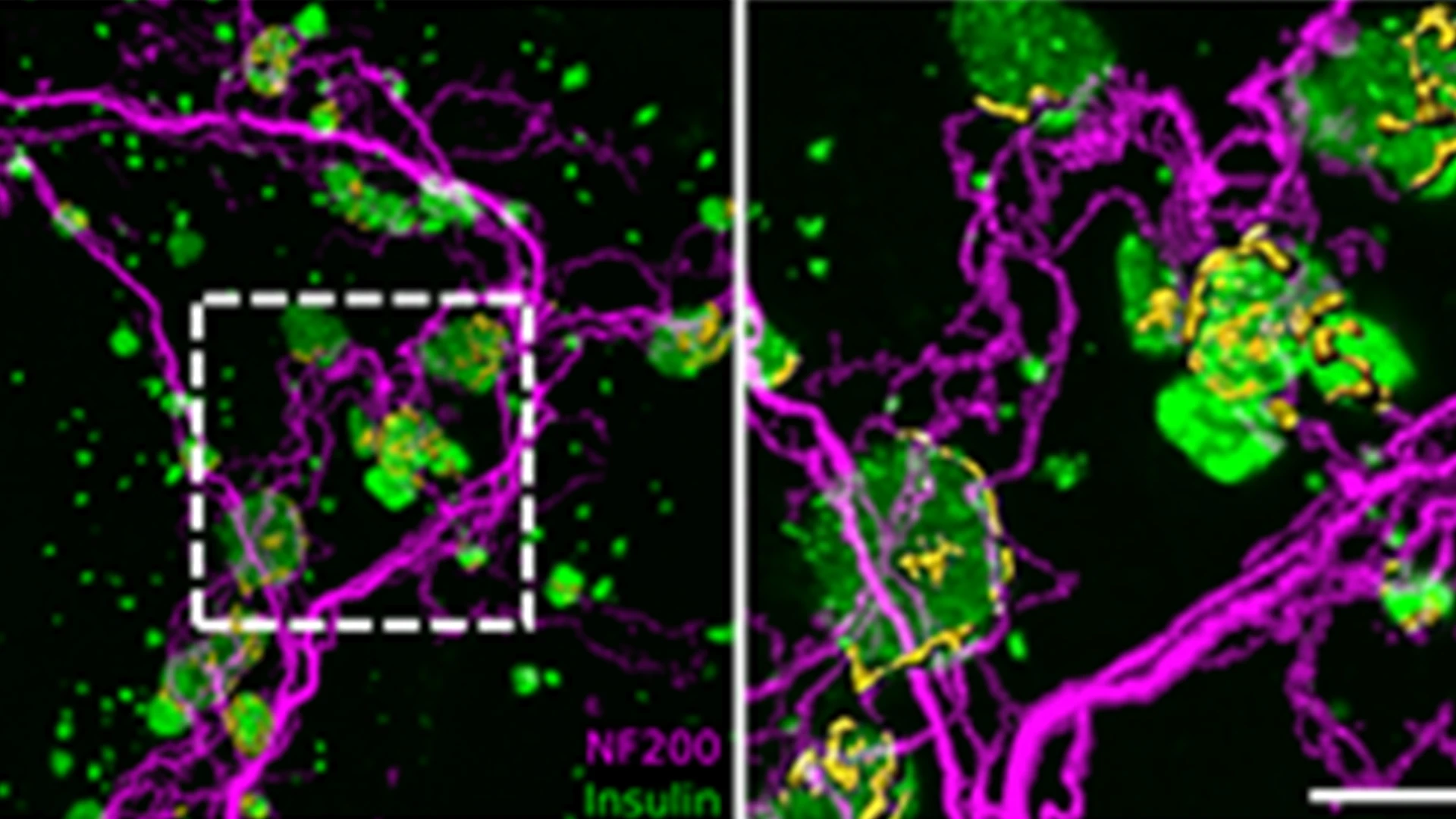
A 3D projection of insulin (green), exocrine innervation (magenta), and innervation within insulin islets (yellow) in the pancreas.

Sarah Stanley, PhD, third from right, with from left, Lucia Alexeyev, a student in Mount Sinai’s CEYE internship program; senior scientists Maria Jimenez-Gonzalez, PhD, and Alexandra Alvarsson, PhD; MD-PhD candidate Rollie Hampton; master’s student Diego Espinoza, and associate researcher Rosemary Li.
Publication:
“A 3D atlas of the dynamic and regional variation of pancreatic innervation in diabetes”
Science Advances, October 2020
Abstract:
Understanding the detailed anatomy of the endocrine pancreas, its innervation, and the remodeling that occurs in diabetes can provide new insights into metabolic disease. Using tissue clearing and whole-organ imaging, we identified the 3D associations between islets and innervation. This technique provided detailed quantification of alpha and beta cell volumes and pancreatic nerve fibers, their distribution and heterogeneity in healthy tissue, canonical mouse models of diabetes, and samples from normal and diabetic human pancreata. Innervation was highly enriched in the mouse endocrine pancreas, with regional differences. Islet nerve density was increased in nonobese diabetic mice, in mice treated with streptozotocin, and in pancreata of human donors with type 2 diabetes. Nerve contacts with beta cells were preserved in diabetic mice and humans. In summary, our whole-organ assessment allows comprehensive examination of islet characteristics and their innervation and reveals dynamic regulation of islet innervation in diabetes.
Takeaway Message, from Sarah A. Stanley, MBBCh, PhD:
We know that neural signals can have a really dramatic effect on the pancreas function and even override what's happening elsewhere in the body. For example, if we switch on a particular nerve or a particular area of the brain, then we can increase or decrease blood glucose, irrespective of whether the animal is hypoglycemic or hyperglycemic before the stimulation.
While we know that neural signals have a dramatic effect, the clinical trials that have looked at nerve stimulation, such as vagal nerve stimulation, have been conflicting and often disappointing.
I think one reason is that we don’t have an in-depth knowledge of the anatomy and the roles of particular pathways. The eventual goal is to pinpoint where we need to intervene, whether that is using a nerve stimulator or giving drugs that activate receptors normally targeted by neurotransmitters.
Vagal nerve stimulators are used for conditions such as migraines or epilepsy. So there are clinical tools available that could potentially be modified to just target the signals from the brain to the pancreas. This would allow us to advance an avenue for controlling pancreas function and hopefully improve glucose control.
Rajbhandari Lab

Team members, from left, Sanil Patel, MS; Prashant Rajbhandari, PhD; and Njeri Sparman.
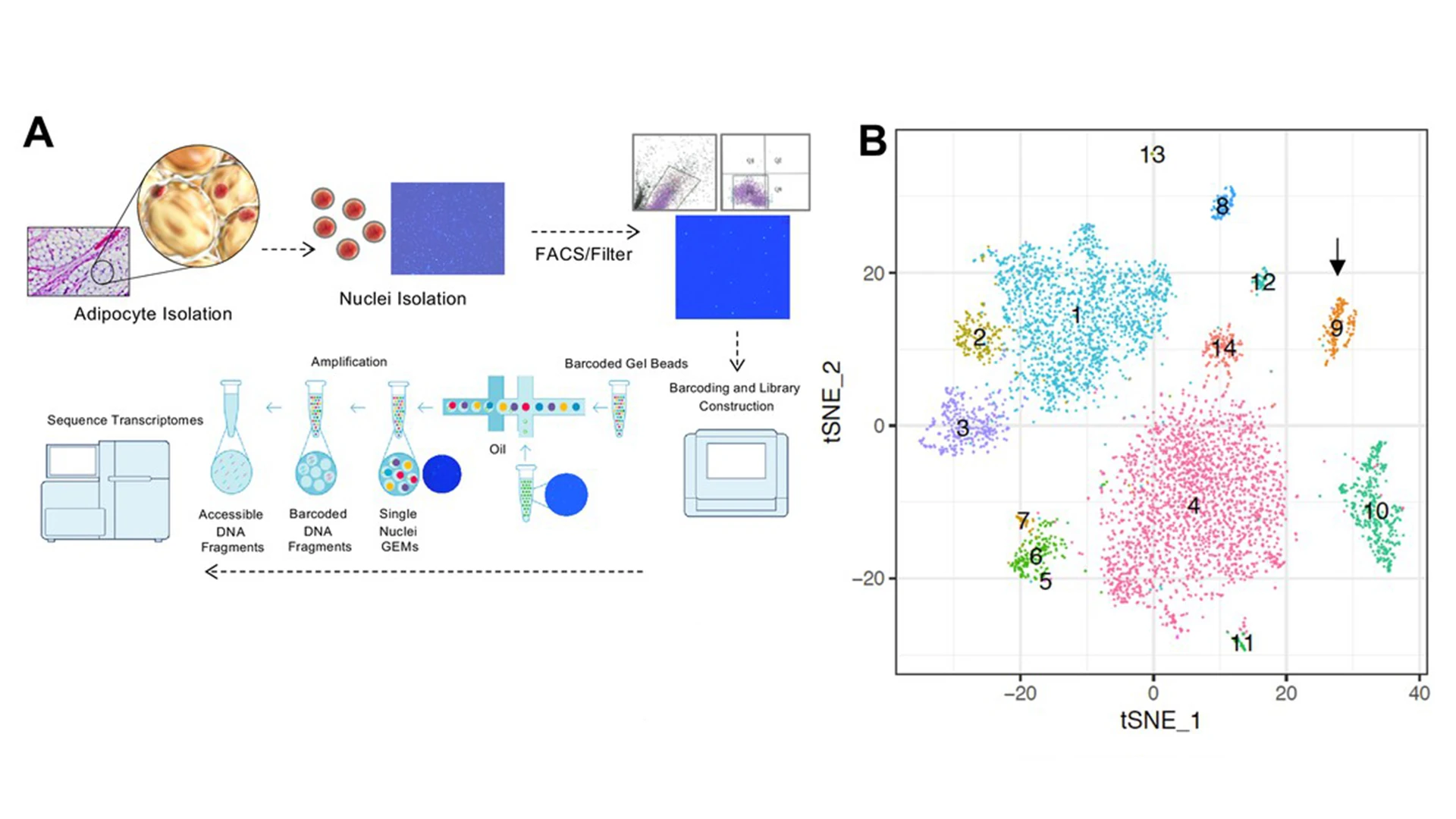
(A) Workflow showing adipocyte nuclei that underwent single nuclei microfluidic partitioning and library preparation followed by sequencing. (B) tSNE-plot showing 17 clusters from 6,000 adipocytes derived from tissues of mice. Each colored dot is an adipocyte assigned to a cluster based on transcriptomic signature.
Publication:
“Single cell analysis reveals immune cell–adipocyte crosstalk regulating the transcription of thermogenic adipocytes” eLife, October 2019
Abstract:
Immune cells are vital constituents of the adipose microenvironment that influence both local and systemic lipid metabolism. Mice lacking IL10 have enhanced thermogenesis, but the roles of specific cell types in the metabolic response to IL10 remain to be defined. We demonstrate here that selective loss of IL10 receptor α in adipocytes recapitulates the beneficial effects of global IL10 deletion, and that local crosstalk between IL10-producing immune cells and adipocytes is a determinant of thermogenesis and systemic energy balance. Single Nuclei Adipocyte RNA-sequencing (SNAP-seq) of subcutaneous adipose tissue defined a metabolically-active mature adipocyte subtype characterized by robust expression of genes involved in thermogenesis whose transcriptome was selectively responsive to IL10R α deletion. Furthermore, single-cell transcriptomic analysis of adipose stromal populations identified lymphocytes as a key source of IL10 production in response to thermogenic stimuli. These findings implicate adaptive immune cell-adipocyte communication in the maintenance of adipose subtype identity and function.
Takeaway Message, from Prashant Rajbhandari, PhD:
The current dogma in the metabolism field is that factors that ameliorate fat tissue inflammation are beneficial for obesity and type 2 diabetes. These factors are classified as anti-inflammatory factors and are thought to act primarily on immune cells.
But what we are finding is a novel and unexpected role of an anti-inflammatory cytokine, Interleukin-10 (IL10) in metabolic homeostasis. Ablation of adipose IL10 signaling in mice improves insulin sensitivity and glucose tolerance, protects against diet-induced obesity, and elicits thermogenesis in fat. We are expanding upon our current observation to preclinical targeting of fat IL10 signaling in obesity and type 2 diabetes.
We also found that the function of a fat cell is much more than just storing lipids or storing excess calories. With the tool we developed, SNAP-seq, we are learning about the spectrum of different types of adipocytes. A huge portion may be storing lipids, but other portions may have specialized function such as converting energy into heat (beige adipocyte) and secreting factors to regulate whole body energy homeostasis.
SNAP-seq is a tool, and no tool is perfect. But it is allowing our team and others to investigate immune-adipocyte crosstalk and the function of different adipocytes. We are not limited to the central dogma—and we are learning more about immune cells and adipocytes that may be therapeutically beneficial to expand upon.