Mount Sinai researchers are developing a novel two-drug approach to treating diabetes that enables the regeneration of insulin-producing pancreatic beta cells. The work has made steady advances since 2015, when the team led by Andrew F. Stewart, MD, Professor of Medicine (Endocrinology, Diabetes and Bone Disease) and Director of the Diabetes, Obesity and Metabolism Institute at the Icahn School of Medicine at Mount Sinai, identified an orally-active drug class that could make beta cells replicate—small-molecule inhibitors of dual-specificity tyrosine-regulated kinase 1A (DYRK1A). The prototype drug, called harmine, was a natural product found in plants. Pure harmine has never been studied carefully in humans, but at doses shown to be effective at inducing rodent and human beta cell regeneration in laboratory animals, it appears to be safe.
To explore its safety in humans, the Mount Sinai team in 2021 received approval from the U.S. Food and Drug Administration to conduct a phase 1 study of harmine in normal, healthy volunteers to see if there are any undesirable central nervous system, cardiovascular, or other effects. The lead investigator in the study is James Murrough, MD, PhD, Associate Professor of Psychiatry and Neuroscience, and Director of the Depression and Anxiety Center for Discovery and Treatment at Icahn Mount Sinai.
“Although harmine is a monoamine oxidase inhibitor (MAOI), no psychoactive properties have been identified in animal studies at the doses we are employing, but animals can’t tell you if they’re hallucinating,” Dr. Stewart notes, adding “We’ll learn from this study what the maximum tolerable dose of harmine is in humans.”
“The enzyme DYRK1A maintains quiescence in adult human beta cells, so it puts the brakes on beta cell proliferation. If you use harmine to take off the brakes, beta cells are now free to proliferate.”
- Andrew F. Stewart, MD
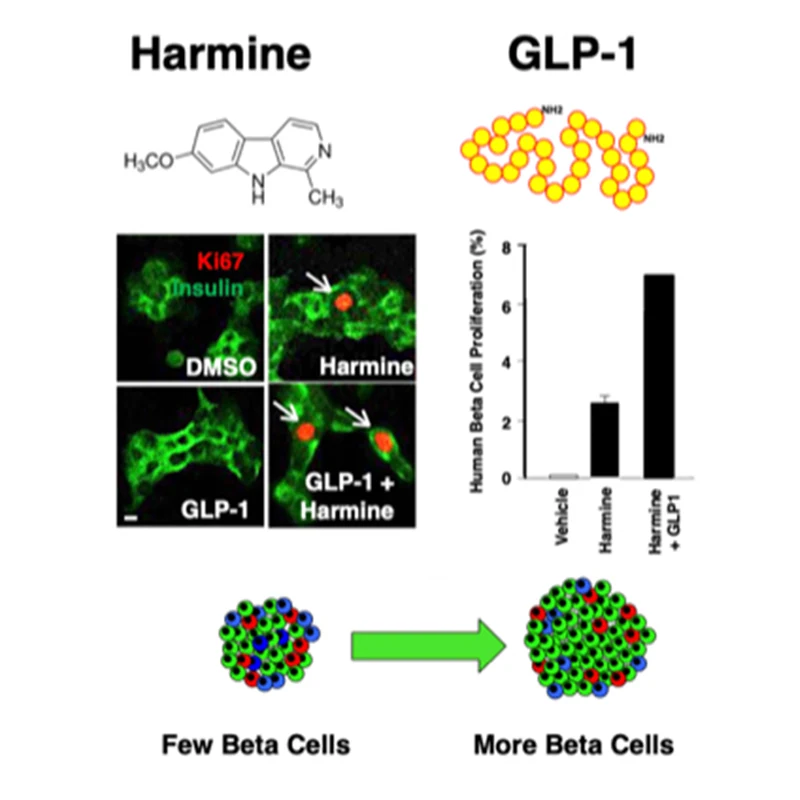
The combination of harmine plus any one of a number of GLP agonists causes a synergistic increase in human beta cell proliferation and functional beta cell mass.
The number of functioning beta cells is reduced in diabetes, by about 90 percent in type 1 and roughly 50 percent in type 2. In both cases, replacing the lost beta cells could restore insulin production, improving glucose control and minimizing the risk of long-term complications. About 643 million people in the world have diabetes according to the World Health Organization. The majority have type 2, while 5 to 10 percent have type 1.
“Everybody with diabetes has a reduced number of beta cells, but all still have some beta cells. Everyone with diabetes needs more beta cells. We set out years ago to find drugs that could help beta cells replicate,” Dr. Stewart says.
Peng Wang, PhD, Professor of Medicine (Endocrinology, Diabetes and Bone Disease), led a search for such drugs using robotic high-throughput screening. In 2015, the team identified harmine and showed that it operates by inhibiting the enzyme Dual-specificity Tyrosine-Regulated Kinase 1A (DYRK1A).
“The enzyme DYRK1A maintains quiescence in adult human beta cells, so it puts the brakes on beta cell proliferation. If you use harmine to take off the brakes, beta cells are now free to proliferate,” Dr. Stewart says.
Next, in order to obtain higher replication rates, the team looked among commercially-available medications used to treat type 2 diabetes. The only drug class they found that had additional proliferative effects on beta cells was the glucagon-like peptide-1 receptor agonist (GLP-1RA) class of injectable or orally administered drugs. These are among the most commonly-prescribed drugs for treating type 2 diabetes. They work by inducing beta cells to secrete insulin in response to elevated blood sugar, and also by inducing satiety, but do not on their own cause beta cells to proliferate.
Dr. Stewart, Dr. Wang, and other Mount Sinai colleagues conducted a study—published in 2020 in Science Translational Medicine—demonstrating that the combination of a GLP-1RA with the DRK1A inhibitor harmine synergistically induced human beta cell replication in human pancreatic islets from cadaveric donors, both ex vivo and transplanted into euglycemic and immunodeficient diabetic mice. The GLP-1RA and harmine combination also normalized glucose in diabetic mice compared to either drug alone or saline.
Beta cell dedifferentiation was not observed, nor were any adverse events in the mouse studies over a one-week period. Longer-term studies will be needed to ensure safety, including to rule out oncogenicity.
Another issue is the underlying autoimmunity in type 1 diabetes, which would still need to be addressed with immunomodulation. But that is also the case with transplants of stem cell-derived islets, another area of active research that has received recent publicity.
Use of the drug combination would be much more scalable, Dr. Stewart says. “Stem cell replacement therapy will be extremely expensive. In contrast, adding one new oral drug to another drug that millions of people are already taking would be affordable and scalable to millions of people with type 1 and type 2 diabetes.”
In addition to Dr. Wang, other key Mount Sinai collaborators in this research include Adolfo Garcia-Ocaña, PhD, Professor of Medicine (Endocrinology, Diabetes and Bone Disease), and Robert DeVita, PhD, Professor of Pharmacological Sciences. “It’s such a depth and breadth of expertise,” Dr. Stewart says. “This is a hugely collaborative effort: team science at its best!”
Featured

Andrew F. Stewart, MD
Professor of Medicine (Endocrinology, Diabetes and Bone Disease) and Director of the Diabetes, Obesity and Metabolism Institute

Sarah Stanley, MBBCh, PhD
Associate Professor of Medicine (Endocrinology, Diabetes and Bone Disease)
