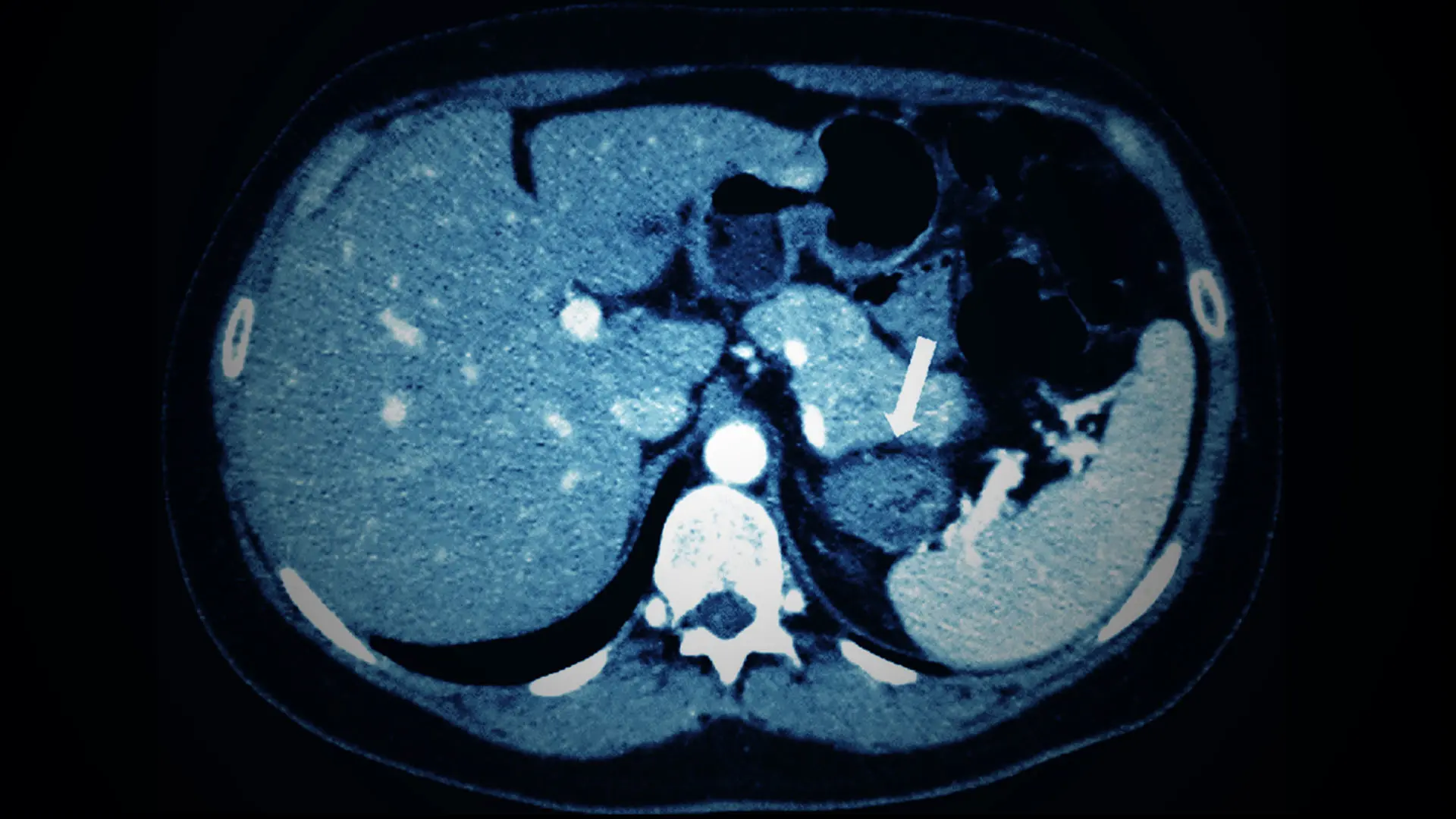
A pilot study led by a Mount Sinai expert in adrenal disorders will examine repurposing an existing therapy for endometriosis to treat mild autonomous cortisol excess (MACE), a condition that is often associated with benign, incidentally found adrenal tumors. Several studies have demonstrated that MACE can be detrimental to blood pressure, weight, bone density, and cardiovascular health.
MACE is present in nearly a third of adrenal incidentalomas—asymptomatic, incidentally found tumors—in women between the ages of 50 and 70, says Alice C. Levine, MD, Director of the Mount Sinai Adrenal Center. Classic Cushing’s syndrome (hypercortisolism) is known to have adverse effects on many organ systems. But recent reports demonstrate that even the modest elevations in cortisol seen in patients with MACE can be just as harmful to the body as full-blown Cushing’s syndrome. Therefore, new approaches to lower cortisol levels in patients with MACE are being tested.
Dr. Levine, Professor of Medicine (Endocrinology, Diabetes and Bone Disease) at the Icahn School of Medicine at Mount Sinai, has proposed an innovative option. Her lab’s research indicates that adrenal tumor development is at least partially driven by adrenocortical progenitor cells that overexpress the luteinizing hormone receptor (LH/hCG-R) in postmenopausal women. LH levels are high in all postmenopausal women not receiving estrogen replacement therapy.
“GnRH antagonists have been around for 40 years, but we believed they could now be an innovative approach to medically treating MACE.”
Alice C. Levine, MD
Dr. Levine and her group postulate that in a subset of postmenopausal women, high LH levels drive adrenal tumorigenesis and produce benign adrenal tumors that overproduce cortisol. Therefore, Dr. Levine proposed repurposing an existing therapy, gonadotropin-releasing hormone (GnRH) antagonists, that lowers LH levels. The oral therapy, elagolix, is made by AbbVie and was approved by the U.S. Food and Drug Administration (FDA) in 2019 for the treatment of endometriosis.
“GnRH antagonists have been around for 40 years, but we believed they could now be an innovative approach to medically treating MACE,” notes Dr. Levine. “So we proposed to AbbVie a pilot study using elagolix to treat autonomous cortisol secretion due to adrenal adenomas in postmenopausal women.” The biopharmaceutical company agreed to the partnership, and an open-label, interventional study with approximately 12 patients from the Mount Sinai Adrenal Center is expected to begin soon.
Specifically, the researchers hope to learn about the effects of elagolix, taken once a day orally, on adrenal adenoma size, cortisol secretion, metabolic parameters, bone turnover markers, and vertebral fracture rates after six months of treatment. A secondary endpoint will be looking at how the medicine impacts mood and quality of life.
“We’re learning that MACE can be very detrimental to a patient’s bones, blood pressure, glucose level, weight distribution, and cardiovascular health, and mortality,” Dr. Levine says. “Unfortunately, physicians are usually at a loss for how to treat this condition. Surgery to remove the adrenal glands is not considered a good option, and the medical treatments currently available have side effects. For those reasons, we believe the GnRH antagonist that we’ll be testing has the potential to be a game-changer for women with incidentally discovered adrenal tumors.”
Question of the Year:
What was the most significant development in your field in 2020?
In the year’s most significant development in my field, the U.S. Food and Drug Administration in March 2020 approved osilodrostat oral tablets for adults with Cushing’s disease who either cannot undergo pituitary gland surgery or have undergone the surgery but still have the disease. This is the first cortisol synthesis blocker specifically approved for the treatment of Cushing’s due to any cause.
- Alice C. Levine, MD
Featured

Alice C. Levine, MD
Director of the Mount Sinai Adrenal Center, and Professor of Medicine (Endocrinology, Diabetes and Bone Disease)