A team at Mount Sinai has created highly purified and functional human thyroid cells from dermal fibroblasts with the ability to secrete thyroid hormone. The novel research, reported in July 2020 in Frontiers in Endocrinology, raises the possibility that an unlimited supply of human induced pluripotent stem cells (iPSCs) could one day be available for patient-specific thyroid regenerative therapy.
“We are very encouraged by the finding that mature adult fibroblasts can be coaxed into human iPSCs and then can form functional thyroid follicular cells that could be suitable for transplantation,” says senior author Terry F. Davies, MD, the Florence and Theodore Baumritter Professor of Medicine (Endocrinology, Diabetes and Bone Disease) at the Icahn School of Medicine at Mount Sinai. “This lays the foundation for patient-specific thyroid stem cells to become powerful tools for biological research as well as a potent source for regenerative medicine.”
Dr. Davies’ laboratory, which has been focused for many years on treating autoimmune thyroid disorders such as Graves’ disease and Hashimoto’s disease, has pioneered the production of human thyroid cells from stem cells. Previous studies led by Dr. Davies have shown that such thyroid stem cells were able to form follicles, and take up radioactive iodine and secrete protein-bound iodine and levothyroxine as evidence of an intact organification process.
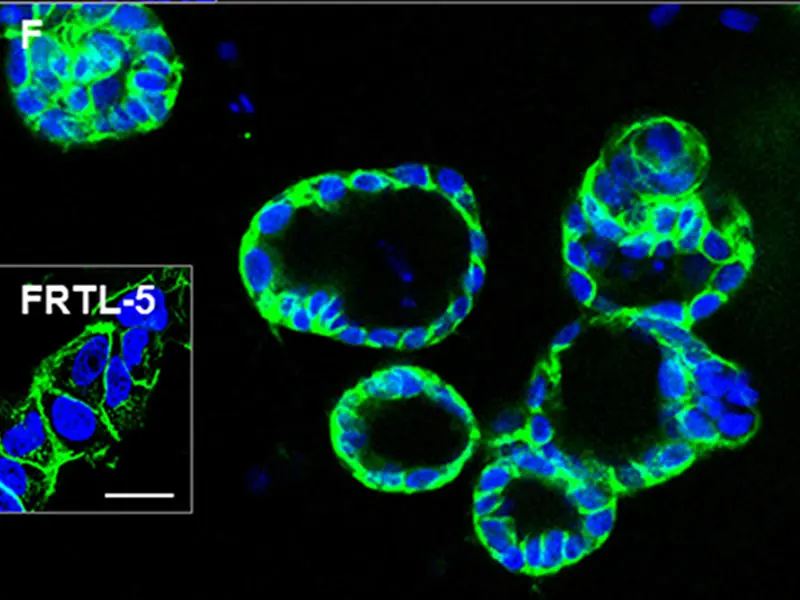
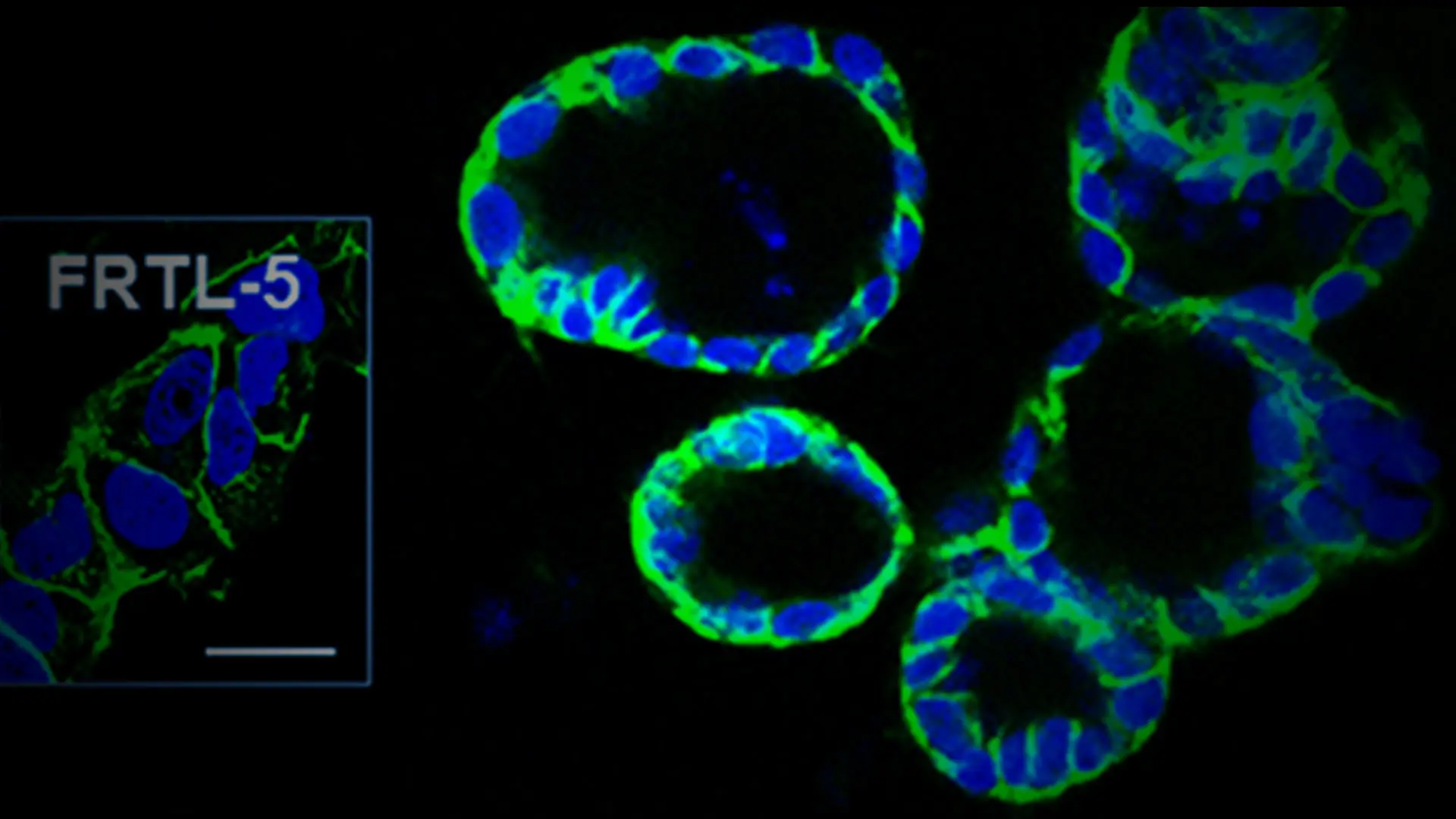
The differentiated cells formed three-dimensional thyroid follicles, shown expressing the thyroid-specific gene TSHR (green) in the membrane. The inset shows staining of rat FRTL-5 thyroid cells.
In this latest study, the team described how it reprogrammed human skin fibroblasts to iPS cells and differentiated them in vitro into thyroid follicular cells by exposure to activin A, a small molecule called ethacridine, and thyroid stimulating hormone (TSH). These cells demonstrated 97 percent purification. “Our characterization of a highly purified population of human thyroid cells augurs well for long-term cell survival, and hopefully for no tumor formation,” says Dr. Davies, an international leader in endocrinology research and treatment. Ongoing transplantation of similar mouse thyroid stem cells into thyroid-deficient mice has shown no tumor formation at six months post transplantation and normalization of thyroid function.
The next step is cell transplantation into primates, Dr. Davies says. This will enable his lab to improve on a technology already in advanced development for making the thyroid stem cells non-immunogenic so that they are not recognized as foreign bodies by animal models. This capability would be critical to eventually creating a procedure not dependent on patient-by-patient stem cell development—an extremely costly and time-consuming prospect—but rather provide an “off-the-shelf” supply of iPS cells that could be used by broad subsets of people with a thyroid deficiency.
These studies are proof of concept for this approach, though many safety concerns must be addressed, including the immunogenic potential of the derived cells and the potential of tumor formation, the research team says, calling for further study with in vivo models.
“The technology has a way to go,” Dr. Davies says, but it holds great promise for the appropriate patients. “For people who have had their thyroid gland removed due to cancer, thyroid cell replacement could be an ideal treatment, as well as for children born with no thyroid gland,” he says. “These therapies might not be that far away, which is why for all of us in the field of thyroid stem cell biology, this is a very exciting time to be involved.”
Question of the Year:
What was the most significant development in your field in 2020?
The biggest challenge in my field of research is understanding the causes of autoimmunity, and the most important development is our increasing ability to link genotypes with phenotypes at all levels, from the cell to the organ to the person.
- Terry F. Davies, MD
Featured

Terry F. Davies, MD
Florence and Theodore Baumritter Professor of Medicine (Endocrinology, Diabetes and Bone Disease)