
This patient had total hair loss five years ago, and is shown before and after treatment
provided by Emma Guttman, MD, PhD.
About two percent of people worldwide have alopecia areata, an autoimmune skin disease that causes hair loss in circular,coin-shaped patches on the scalp. This condition often first appears in childhood, with 40 percent of people developing symptoms before the age of 40.
While it is not a life-threatening condition, it can be devastating to live with this disease, says Emma Guttman, MD, PhD, Waldman Professor and System Chair of Dermatology and Immunology at the Icahn School of Medicine at Mount Sinai. Up until 2022, there were no treatments approved by the Food and Drug Administration (FDA) for severe alopecia areata. “Many patients simply just learned to live with it,” Dr. Guttman adds.
Thankfully, new drugs for alopecia areata have begun to gain FDA approval, including one in 2023. Today, Dr. Guttman and her team are on a mission to create even more options through Mount Sinai’s Alopecia Center of Excellence.
Launched in March 2022 through the generous support of the Pure family, the Center is the first of its kind—the only centralized center in the country that brings together compassionate patient care, the most advanced treatments, and clinical trials with novel drugs.
“Mount Sinai is uniquely positioned to take the lead on groundbreaking research in alopecia areata,” says Benjamin Ungar, MD, Director of the Center. This is due to two main reasons, he notes: “The motivated patients who come to Mount Sinai seeking help, as well as a fantastic team of researchers working together to identify new treatment targets and design clinical trials to establish new treatments.” There are exciting developments to come out of the Center this year.
Addressing Alopecia Areata in Children
Pediatric alopecia areata affects about 1 in 1,000 children and teens. While the condition generally presents similarly in children and adults, an earlier onset in life can be associated with a worse prognosis, in terms of both severity and response to treatment. “This condition has a tremendously negative psychological impact on many people who suffer from it, and those difficulties can be unique in the pediatric population compared with adults,” adds Dr. Guttman. About half of teens report that they’re embarrassed by their hair loss, for example, and another 40 percent say that they’ve been bullied, according to the National Alopecia Areata Foundation.
That is why Dr. Guttman is particularly excited about a prestigious grant the Center expects to be awarded from the National Institutes of Health in 2024 to study the use of dupilumab in children and teens aged 6-17 with severe alopecia areata. “It offers promise and hope for a population in need,” Dr. Guttman says.
Dupilumab is a type of biologic drug called a monoclonal antibody that targets two specific proteins in the body, interleukin-4 (IL-4) and interleukin-13 (IL-13) to regulate the immune response. It was approved to treat atopic dermatitis, or eczema, in adults in 2017. Dr. Guttman first uncovered the link between dupilumab and alopecia areata when she discovered that adult patients with both alopecia and atopic dermatitis who were given the drug also experienced hair regrowth.
“We aim to evaluate dupilumab, which has been shown to be extremely safe in atopic dermatitis and other conditions, as an effective treatment for children with alopecia areata,” says Dr. Guttman. “We understand that it’s important to treat the disease within a few years of its onset, as the longer it goes untreated, the less likely an excellent response to treatment will occur.” The trial will also help to provide more mechanistic insights into alopecia areata, which may spur progress in future research.
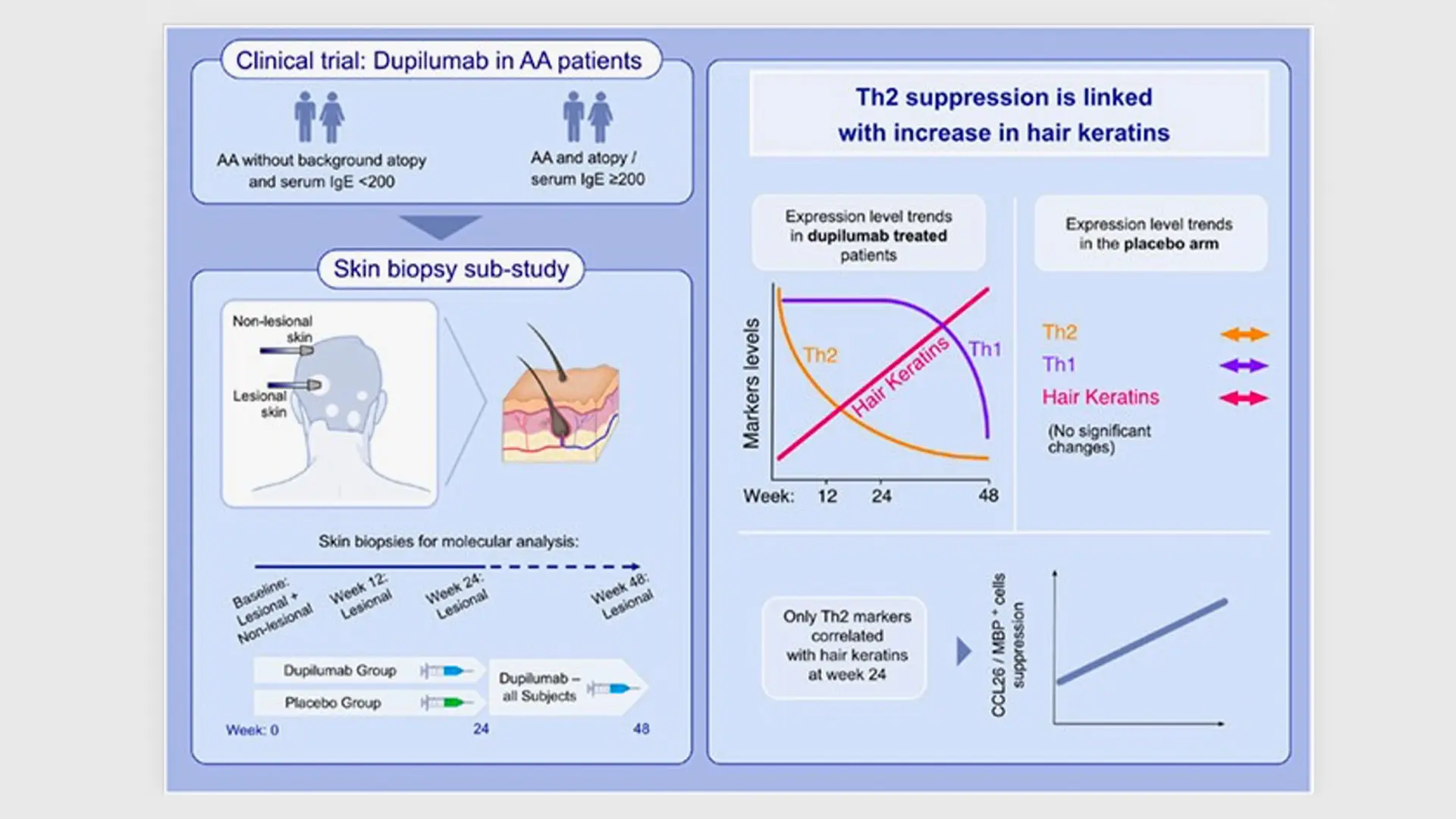
This study shows that scalp biomarkers provide evidence of dupilumab efficacy in AA, detected even prior to clinical response, with exclusive correlations between early suppression of Th2 markers and increased hair keratins. These findings strengthen previous reports suggesting a possible role for Th2 cytokines as AA drivers.
New Drugs Emerging
The Center was also involved in clinical trials for two new drugs to treat alopecia areata: ritlectinib, which got FDA approval in June 2023, and deurxuolitinib, which is expected to get FDA approval in the first half of 2024. “It’s been an incredible journey for us at Mount Sinai,” says Ester Del Duca, MD, an instructor in the Laboratory of Inflammatory Skin Diseases at Icahn Mount Sinai. “It’s given us firsthand insights into how these treatments work in real-life patient situations.”
In addition, the Center is looking for innovative new drug targets for treatment of alopecia areata that Dr. Guttman and her team are particularly excited about, which stem directly from research done in the lab and may lead to treatments that are approved or in trials for other indications.
As the Center moves into 2024, it’s well positioned to be on the cutting edge of research. “All of this involvement places us at the forefront of medical innovation, especially in treating conditions like alopecia areata, where some patients don’t respond to existing treatments,” stresses Dr. Guttman. “We need fresh solutions and a fresh look at alopecia. The more drugs we study and develop, the better it is for everyone. We also need to better understand the association between alopecia and allergic diseases, to allow for the development of treatment strategies that can work on both patient populations.”
