A clinical study led by Mount Sinai offers strong evidence that aggressive lipid lowering with a proprotein convertase subtilisin kexin type 9 inhibitor (PCSK9i), along with a statin, can significantly reduce that threat and potentially help doctors identify patients who would benefit most from intensification of treatment to change their coronary plaque morphology and composition.
The findings were presented by principal investigator Annapoorna S. Kini, MD, Director of the Cardiac Catheterization Laboratory at The Mount Sinai Hospital, as a late-breaking clinical trial at the American College of Cardiology/World Congress of Cardiology meeting in New Orleans in March 2023.
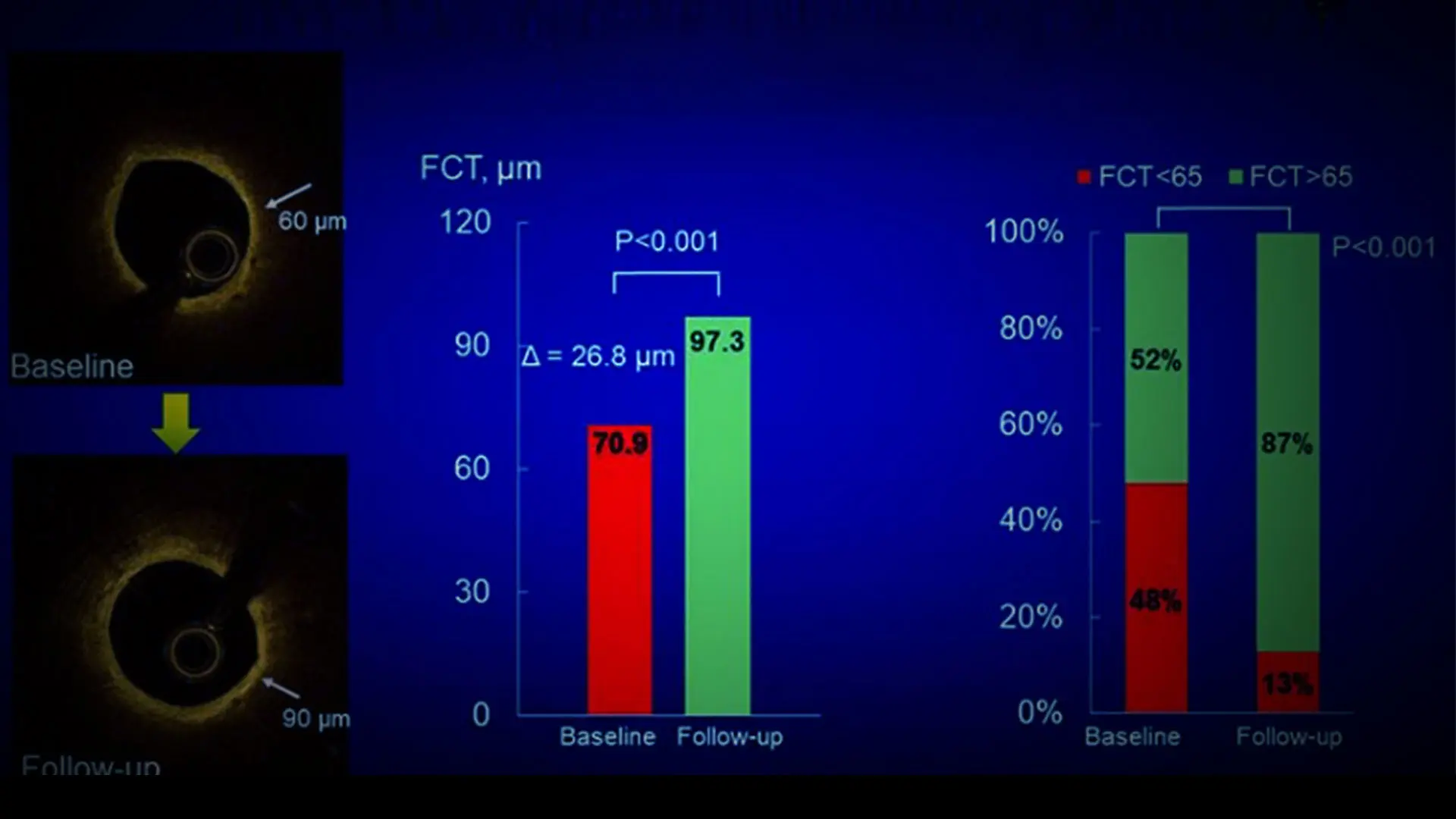
The study, known as Yellow III, used advanced multimodality imaging to show favorable plaque characteristics after a 26-week regimen of evolocumab, including substantial reductions in total cholesterol, LDL cholesterol, and total/HDL cholesterol ratios. More specifically, the investigation showed a significant increase in the minimum fibrous cap thickness (FCT) through optical coherence tomography (OCT), reduction in lipid core burden index at the maximal 4-mm segment (maxLCBI4mm) through near-infrared spectroscopy, and reduction in atheroma volume through intravascular ultrasound in angiographically nonobstructive lesions.
Prior studies have established the ability of PCSK9 inhibitors—injectables that block PCSK9 proteins from breaking down LDL receptors—to reduce residual cardiovascular risk in statin-treated patients. As a result, the 2018 American College of Cardiology/American Heart Association cholesterol guidelines recommended the use of PCSK9 inhibitors in patients with stable CAD if sufficient LDL-lowering was not achieved on maximally tolerated doses of statins.
In the Yellow III trial, 137 patients scheduled for elective coronary angiography were prescribed maximum-dosage statin therapy for at least four weeks before undergoing multimodality intracoronary imaging. They were then given evolocumab (140 mg) every two weeks for 26 weeks and reimaged to assess changes in plaque morphology and composition.
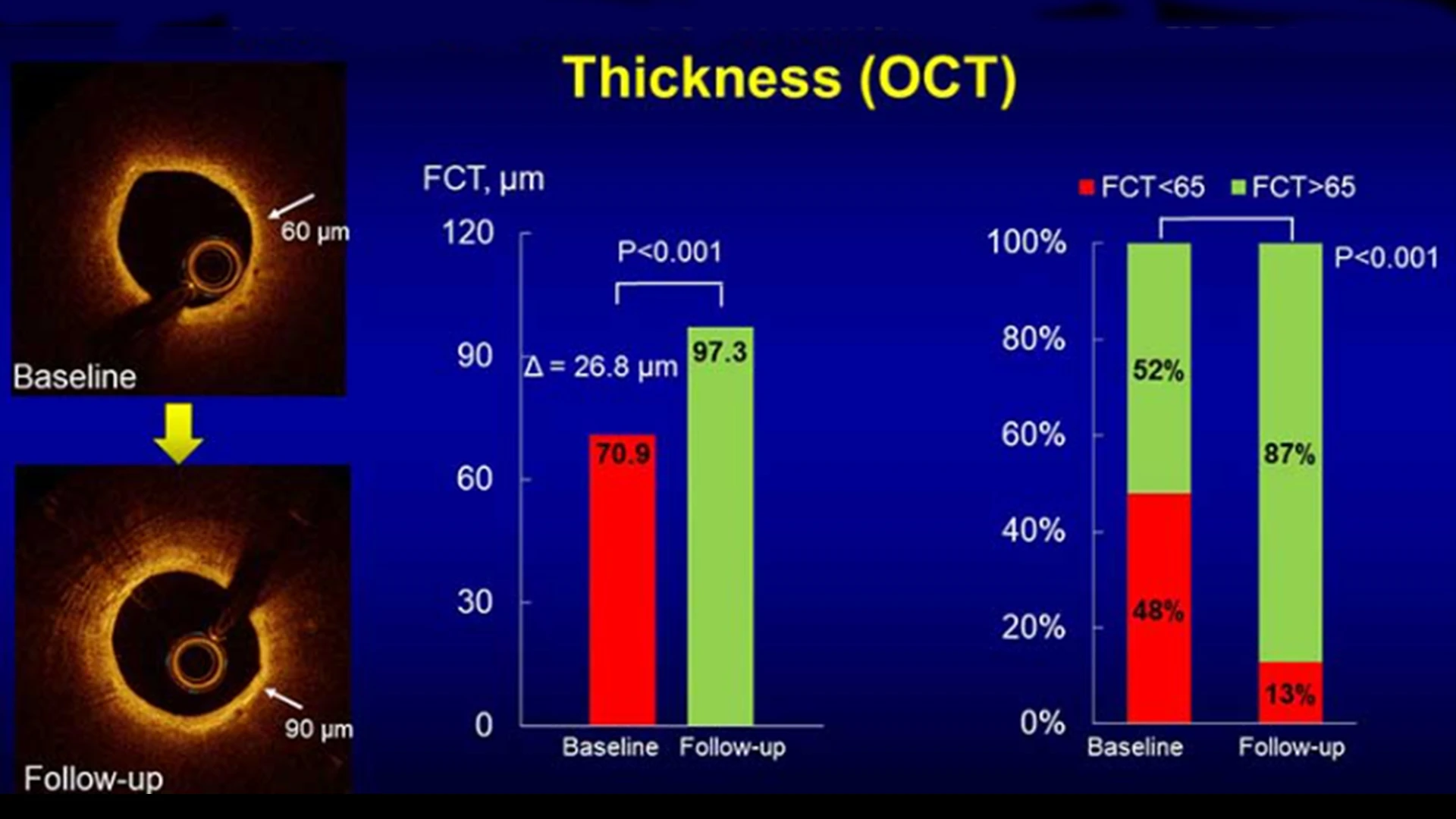
The investigation showed a significant increase in the minimum fibrous cap thickness through optical coherence tomography (OCT) imaging. Thicker fibrous caps are associated with more stable plaques that are less prone to rupture and subsequent adverse cardiac events.
“By using all three modalities for the first time in a study of this type we were able to demonstrate a measurable improvement in fibrous cap thickness, as well as in plaque volume,” says Dr. Kini, Zena and Michael A. Wiener Professor of Medicine (Cardiology) at the Icahn School of Medicine at Mount Sinai. “In addition, blood samples were drawn to enable us to conduct a gene expression analysis of peripheral blood mononuclear cells. This will help us uncover through ongoing research the molecular mechanisms responsible for beneficial changes in atherosclerotic lesions of patients treated with evolocumab.”
Prior studies have established the ability of PCSK9 inhibitors—injectables that block PCSK9 proteins from breaking down LDL receptors—to reduce residual cardiovascular risk in statin-treated patients. As a result, the 2018 American College of Cardiology/American Heart Association cholesterol guidelines recommended the use of PCSK9 inhibitors in patients with stable CAD if sufficient LDL-lowering was not achieved on maximally tolerated doses of statins. In the Yellow III trial, 137 patients scheduled for elective coronary angiography were prescribed maximum-dosage statin therapy for at least four weeks before undergoing multimodality intracoronary imaging. They were then given evolocumab (140 mg) every two weeks for 26 weeks and reimaged to assess changes in plaque morphology and composition.
The gene expression analysis of peripheral blood mononuclear cells was a particularly important part of the Yellow III study because it could potentially lead to the development of biomarkers able to predict which patients would benefit the most from different approaches to lipid lowering. Researchers found that fibrous cap thickness did not improve in 20 percent of patients. The hope is that a genotypic characterization of patient response will ultimately reveal which patients should remain on statins, which should be put on a PCSK9 inhibitor, and which might benefit from combination therapy.
“We believe studies like ours can help physicians personalize therapies for their patients with coronary artery disease,” says Dr. Kini. “The first step could well be a recommendation for lifestyle modification, like exercise and diet. But it is important for cardiologists to know who could also benefit from the addition of a high-intensity PCSK9 inhibitor, particularly in the case of statin-treated patients with multiple risk factors.”
Principal investigator Annapoorna S. Kini discusses the Yellow III trial, including its history, the baseline characteristics of enrolled patients, and its most significant findings.
Featured

Annapoorna S. Kini, MD
Director of the Cardiac Catheterization Laboratory at The Mount Sinai Hospital, and the Zena and Michael A. Wiener Professor of Medicine

Samin K. Sharma, MD
Director of Interventional Cardiology, The Mount Sinai Hospital, and Anandi Lal Sharma Professor of Medicine in Cardiology