In the century since the world’s first successful heart valve repair, millions of patients have received a new lease on life through aortic, mitral, and pulmonary valve procedures. Now, for the first time, specialists can also offer a full range of effective therapies for the tricuspid—often known as “the forgotten valve”—including tricuspid transcatheter edge-to-edge repair (T-TEER) and transcatheter tricuspid valve replacement (TTVR).
The Mount Sinai Fuster Heart Hospital’s leadership role in the TRILUMINATE pivotal trial helped prove T-TEER’s safety and efficacy, which led the Food and Drug Administration to approve the Abbott TriClipTM device in April 2024. Mount Sinai is now the highest-volume center for mitral and tricuspid T-TEER procedures in New York, and among the highest in the United States. The hospital is also regularly performing TTVR on patients who need entirely new tricuspid valves, using the Edwards Lifesciences’ Evoque Tricuspid Valve, the first transcatheter replacement valve to receive FDA approval, in February 2024.
TriClip was found to be safe and effective in the full randomized cohort of the TRILUMINATE Pivotal trial with significant reduction in tricuspid regurgitation (TR) and improvements in six-minute walk distance and health status, according to a study published in December 2024 in the Journal of the American College of Cardiology. Rates of all-cause mortality or tricuspid valve surgery and heart failure hospitalizations through one year were not reduced by T-TEER.
“For the first time, we’ve demonstrated an objective, statistically significant increase in six-minute walk distance, confirming functional benefits beyond subjective feelings of improvement,” says Gilbert H.L. Tang, MD, MSc, MBA, Surgical and Academic Director of the Structural Heart Program, Director of Mitral and Tricuspid Structural Interventions at the Mount Sinai Health System, and Professor and Vice Chair of Innovations in the Department of Cardiovascular Surgery at the Icahn School of Medicine at Mount Sinai.

Members of the team at Mount Sinai Fuster Heart Hospital, from left: Lucy Safi, DO, FACC, FASE, FSCAI; Sahil Khera, MD, MPH; Gilbert H.L. Tang, MD, MBA, MSc; Stamatios Lerakis, MD, PhD; David H. Adams, MD; Samin K. Sharma, MD; Annapoorna S. Kini, MD; Parasuram Krishnamoorthy, MD; and Jin Kang, MD.
In addition to medical therapy and tricuspid valve repair surgery, these new transcatheter options are good news for the roughly 1.3 million Americans suffering tricuspid regurgitation, which, left unaddressed, can damage the right ventricle, liver, and kidneys; cause ankle edema; and lead to heart failure. In fact, many patients are not diagnosed with TR until they have already suffered significant damage.
The national Co-Principal Investigator on the TRILUMINATE Pivotal trial was David H. Adams, MD, the Marie-Josée and Henry R. Kravis Professor and Chair of Cardiovascular Surgery at the Icahn School of Medicine at Mount Sinai, and Cardiac Surgeon-in-Chief for the Mount Sinai Health System. As the trial’s surgical lead, he partnered with cardiologist and co-Principal Investigator Paul Sorajja, MD, the Roger L. and Lynn C. Headrick Family Chair of the Valve Science Center at the Minneapolis Heart Institute Foundation, and Director of the Minneapolis Heart Institute’s Center for Valve and Structural Heart Disease.
Dr. Adams notes that the full range of treatment options for TR, whether surgical or transcatheter, now “improve the quality of life for a patient population that previously confronted substantial morbidity and poor long-time survival rates.”
Patients for whom surgery poses intermediate or greater risk qualify for these new transcatheter procedures, representing a shift in the standard of care. And for patients experiencing severe TR, when medical management and surgery may not be as effective or pose too great a risk, these new alternatives could be life-changing.
“If patients are too sick to undergo surgery or benefit from diuretics, transcatheter tricuspid repairs or replacements offer breakthrough options,” says Dr. Tang, who in April 2024 led the team performing one of the first T-TEER procedures in the United States. “The tricuspid used to be called the ‘forgotten valve’ because there was no good way to fix it short of surgery. Now we can offer a full range of effective treatment options, including transcatheter options, so that even the sickest patients with severe TR have a good chance of improving their quality of life.”
Along with Dr. Tang, the Mount Sinai team that performs these procedures is led by Annapoorna S. Kini, MD, Director of the Mount Sinai Fuster Heart Hospital’s Cardiac Catheterization Laboratory, and Director of the Interventional Structural Heart Program at the Mount Sinai Health System. “As one of the nation’s leaders in providing patients with the latest in transcatheter therapies, we can now welcome early referrals of really sick patients, because we know we are in a strong position to help them,” Dr. Kini says.
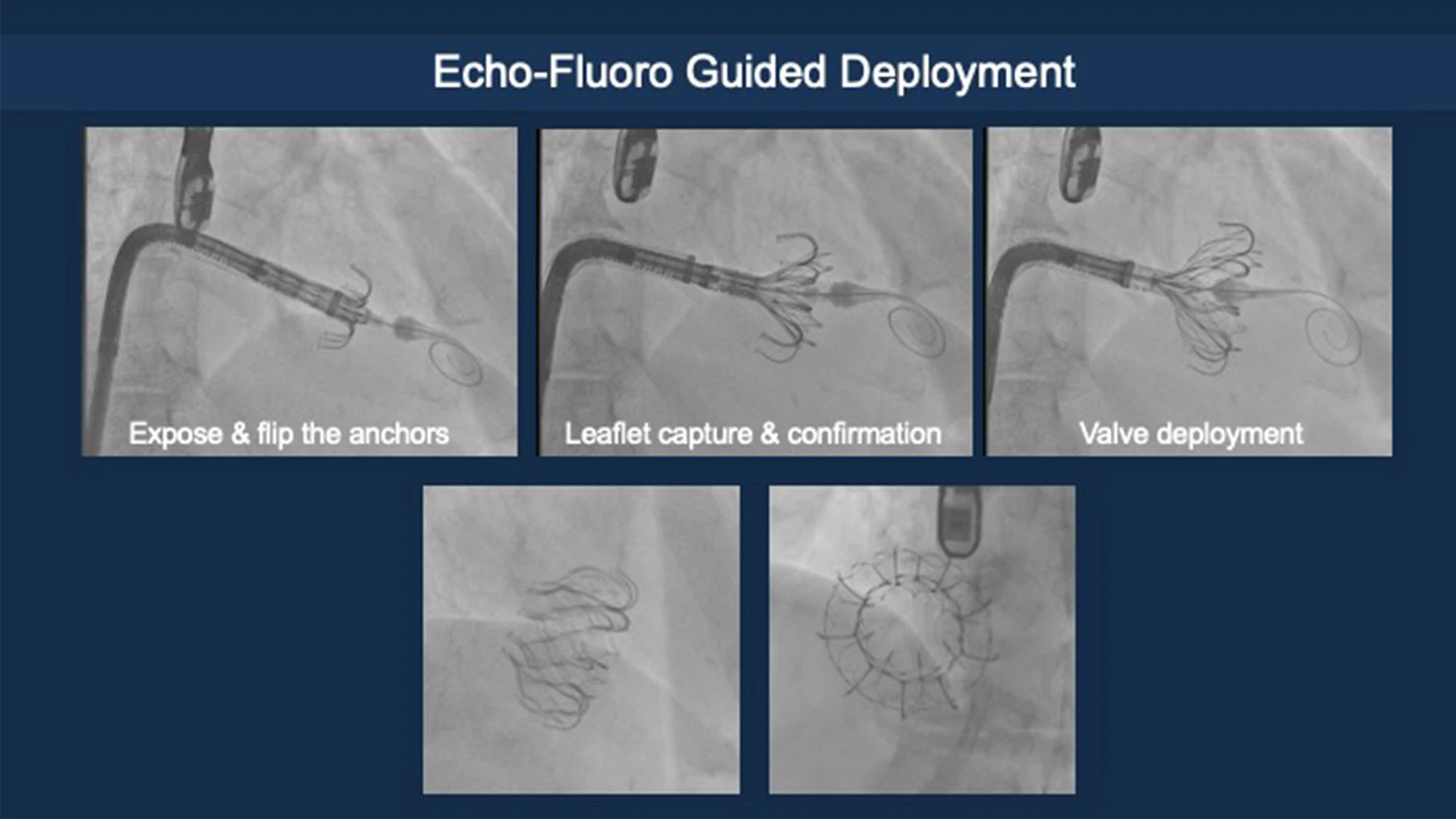
Deployment of the Evoque device, guided by echo-fluoro imaging.
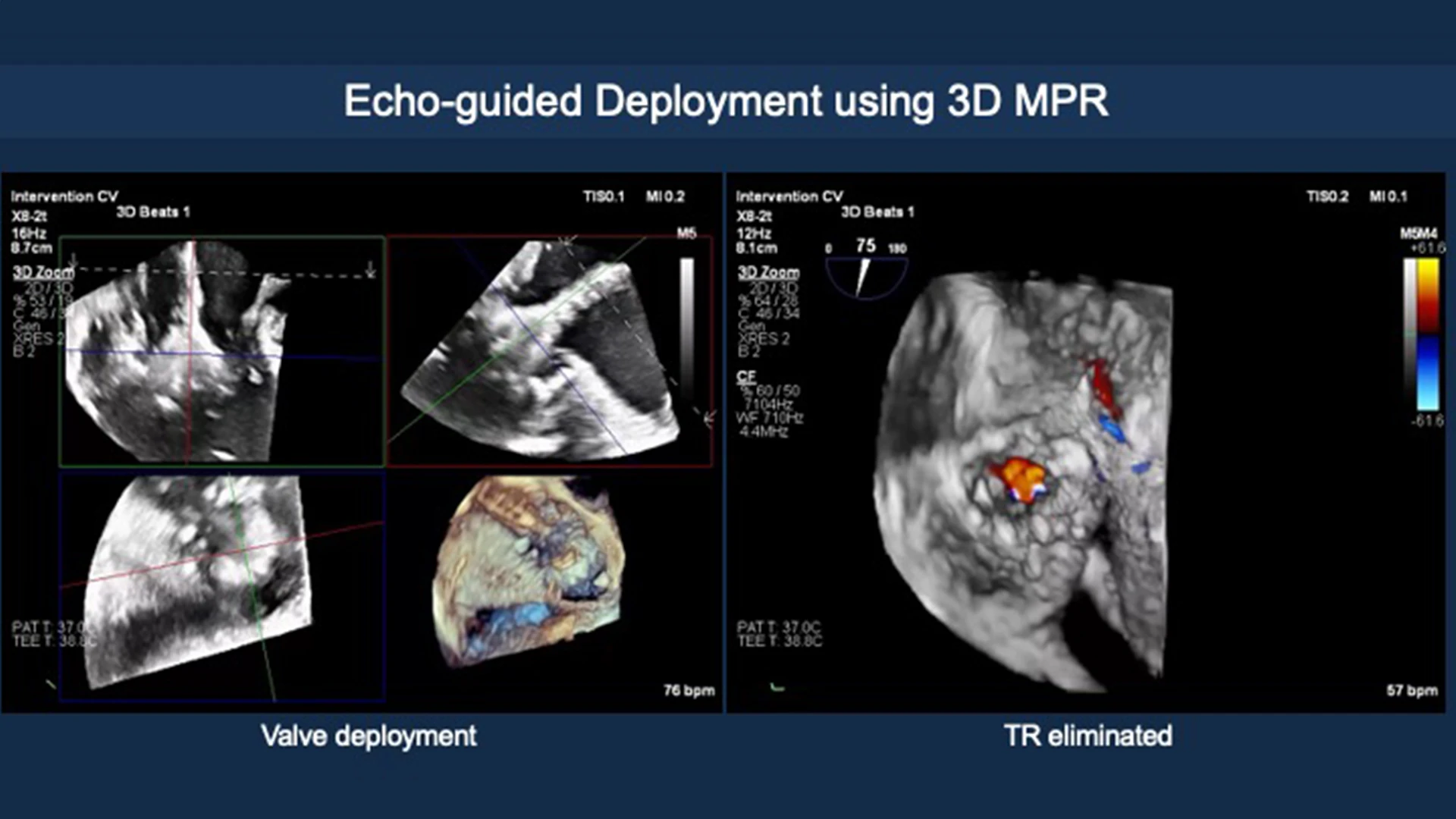
Deployment using 3-D multiplanar reconstruction.
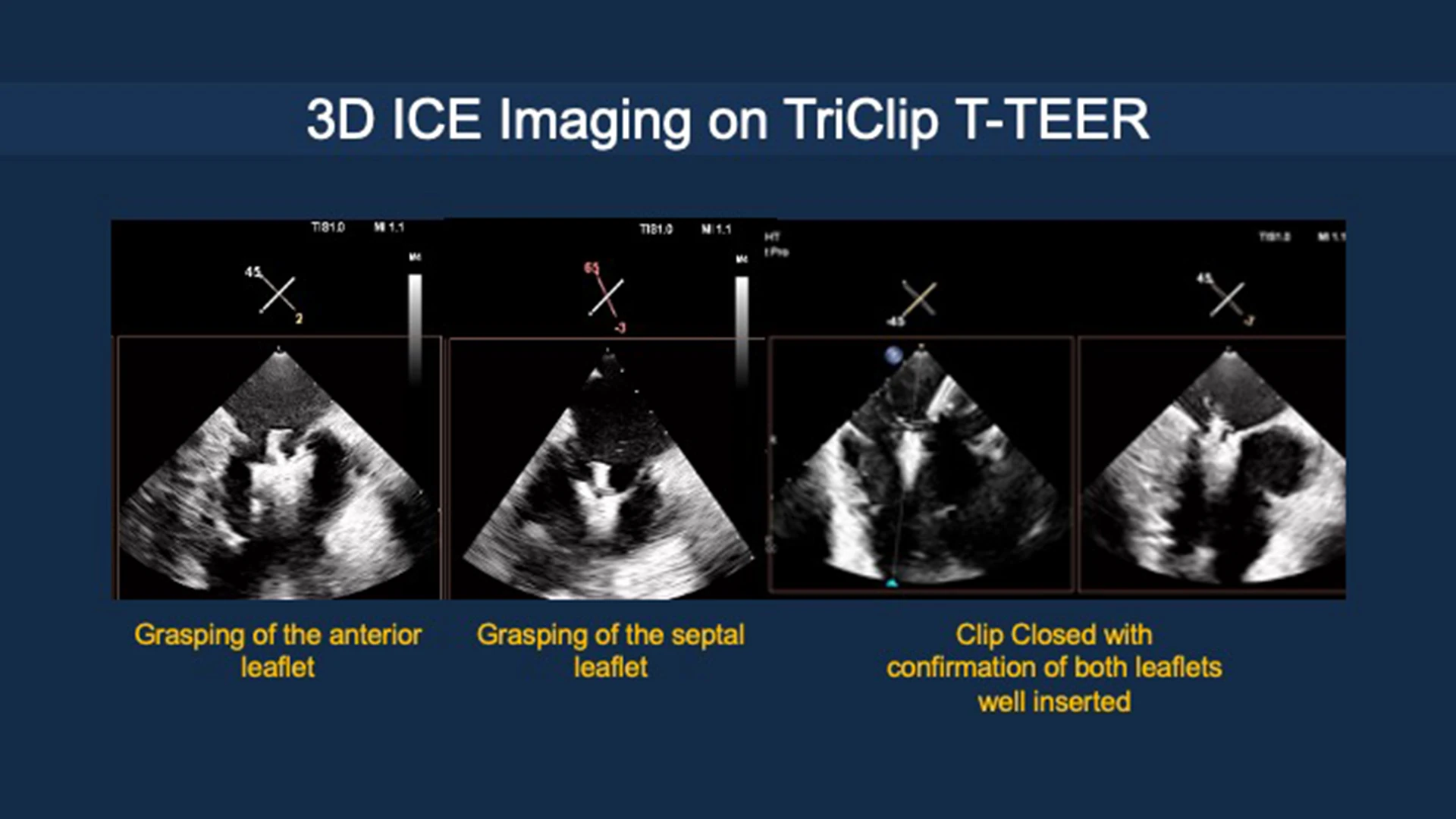
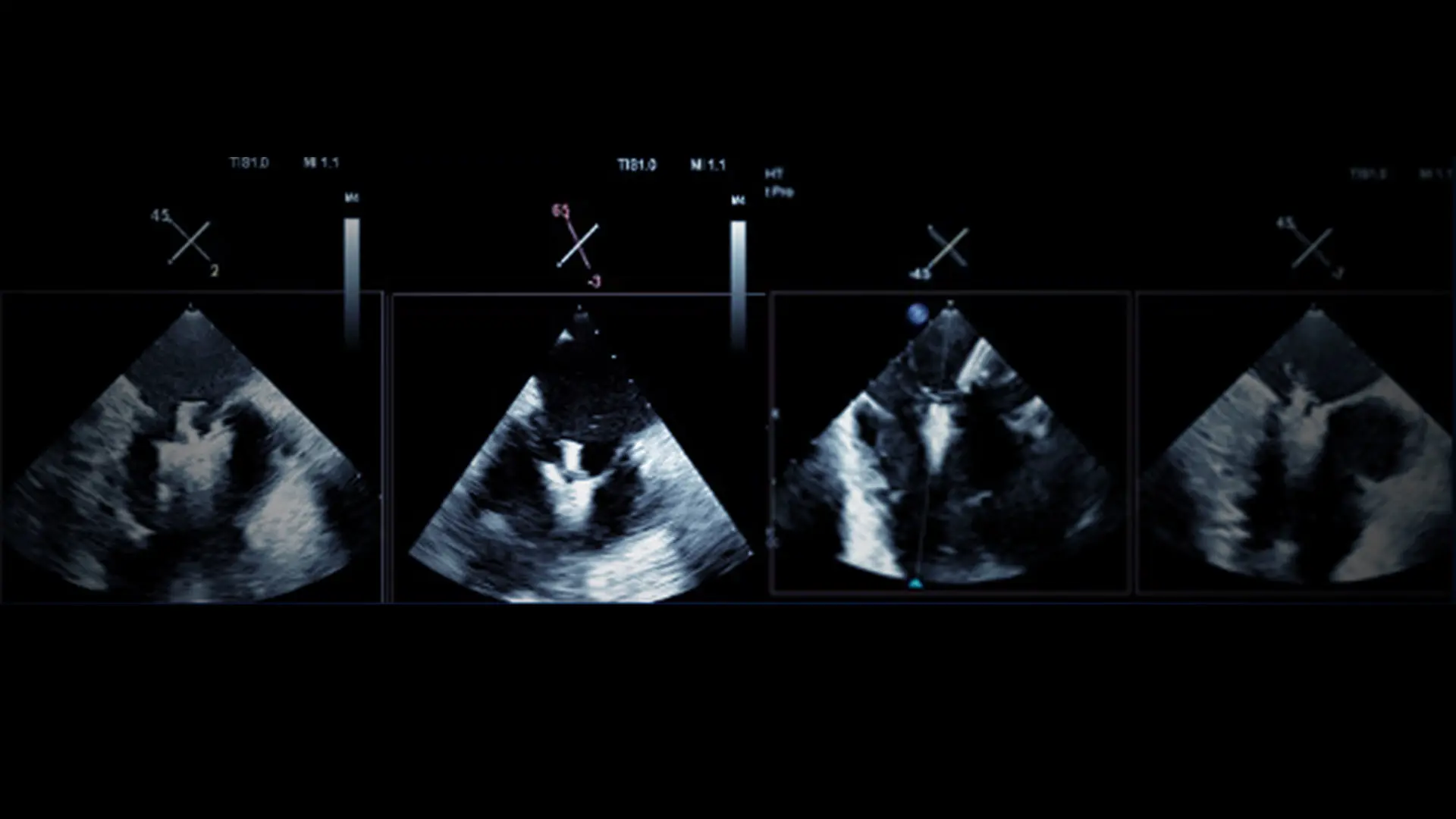
3-D imaging on tricuspid transcatheter edge-to-edge repair.
Samin K. Sharma, MD, Director of the Interventional Cardiology and Cardiovascular Clinical Institute for the Mount Sinai Health System, says, “This intervention could be a life-saver for many patients who can’t undergo open heart surgery. T-TEER and TTVR allow us to treat patients who have failed medical therapy much earlier in the disease cycle, and more effectively.”
As reported in the The New England Journal of Medicine, T-TEER was safe in more than 98 percent of patients who remained free of major adverse events at 30 days, based on data from the TRILUMINATE trial. Similarly, as presented at TCT 2023, the TRISCEND II trial data indicated “favorable safety and effectiveness outcomes for the Evoque tricuspid replacement valve.”
“Patient recoveries have been consistently remarkable,” Dr. Tang says. “We’re demonstrating that the tricuspid TEER technology can significantly impact health outcomes for patients with tricuspid valve disease who experience fatigue, shortness of breath, irregular heart rhythms, swelling and organ dysfunction.”
In June 2024, one of those patients was an octogenarian suffering severe tricuspid regurgitation so debilitating that he had to fly the 94 miles from Philadelphia to New York for treatment at the Mount Sinai Fuster Heart Hospital. Weeks later, thanks to a successful T-TEER procedure, he was playing baseball with his grandchildren again.
As Dr. Kini notes: “Patients with tricuspid regurgitation are often quite sick, with frequent hospital admissions for heart failure symptoms, and see limited benefits from medications other than diuretics. Therefore, widespread appropriate use of these new transcatheter procedures has the potential to change the prognosis of this disease entity.”
Doctors performing the T-TEER procedure implant a TriClip device via a catheter, through the patient’s femoral vein. The TriClip improves the closure of the tricuspid valve’s leaflets, significantly reducing the backflow of blood to the right atrium. TriClip leverages the technology and robust body of evidence from Abbott’s MitraClipTM, which is a widely used, minimally invasive option to repair leaky mitral valves.
For patients whose anatomy may be challenging for T-TEER, the Evoque valve replacement can be an effective treatment. Mount Sinai is one of the first hospitals in the United States outside the TRISCEND II trial to offer this groundbreaking therapy to patients with severe TR.
“What we see in patients undergoing TTVR is the immediate near abolishment of TR, which is remarkable,” Dr. Tang says. “They do require admission prior to and after the procedure to condition the right ventricle to accept the new valve, and there is a risk of needing a pacemaker and temporarily be on blood thinners after the procedure. Once the right heart recovers from the TTVR procedure, they feel great and continue to do well because there is almost no TR remaining.”
To refer a patient with tricuspid regurgitation to the Mount Sinai Fuster Heart Hospital, please call 212-659-6820.