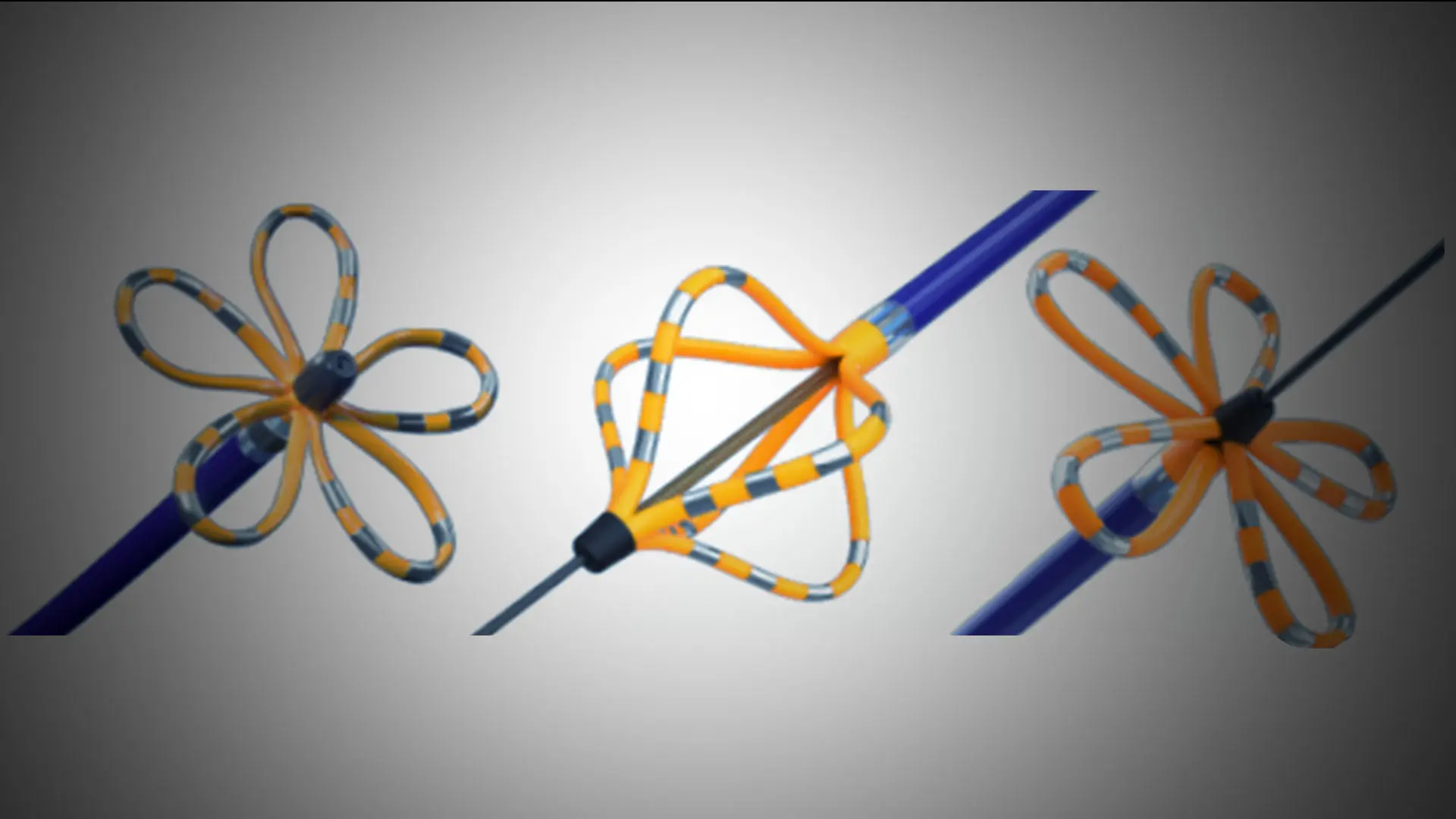
Pulsed field ablation (PFA) is safe for treating patients with common types of atrial fibrillation (AF) and “has the potential to be transformative for the management of patients with AF,” according to the largest study of its kind on this new technology, led by Vivek Y. Reddy, MD, Director of Cardiac Arrhythmia Services, Mount Sinai Fuster Heart Hospital. The MANIFEST-17K international study, published in July 2024 in Nature Medicine, is the first to show important safety outcomes with PFA in a large patient population, including no significant risk of esophageal damage.
“MANIFEST-17K provides confidence that, unlike conventional thermal ablation, PFA with the pentaspline catheter does not cause the most feared complication of AF ablation—esophageal damage—nor does it cause pulmonary vein stenosis or persistent injury to the diaphragm,” says Dr. Reddy, senior author of the study, and the Leona M. and Harry B. Helmsley Charitable Trust Professor of Medicine in Cardiac Electrophysiology, and Professor of Artificial Intelligence and Human Health at the Icahn School of Medicine at Mount Sinai. “This study found that other general complications were also rare, including pericardial tamponade occurring in approximately 1 in 200 patients, stroke in 1 in 1,000, and death in less than 1 in 1,000 patients. Given the relative novelty of pulsed field ablation, these are important safety outcomes.”
The retrospective, observational MANIFEST-17K study analyzed 17,642 patients with paroxysmal or persistent AF after undergoing PFA procedures at 106 centers in 2021. Procedures were performed with the pentaspline PFA catheter, the most commonly used PFA catheter worldwide. Researchers found no energy-specific complications among patients, including no esophageal damage, pulmonary vein stenosis, or persistent phrenic nerve injury. There was a 1 percent overall major complication rate, and more specifically, rates of 0.36 percent for pericardial tamponade, 0.30 percent for major vascular complications, 0.12 percent for stroke, and 0.03 percent for death. Unexpected complications of PFA were coronary arterial spasm (0.14 percent) and hemolysis-related acute renal failure necessitating hemodialysis (0.03 percent).
“PFA with the pentaspline catheter does not cause the most feared complication of AF ablation—esophageal damage—nor does it cause pulmonary vein stenosis or persistent injury to the diaphragm.”
Vivek Y. Reddy, MD
“While we should continue to remain vigilant to identify any other rare complications of PFA that may be identified in the future, these favorable safety outcomes in more than 17,000 patients increase our confidence in the use of this PFA catheter technology,” Dr. Reddy says.
In January 2025, Dr. Reddy described results of the Advantage AF trial, the first large prospective study of PFA to treat persistent atrial fibrillation, in a late-breaking presentation at the AF Symposium in Boston. The study found favorable outcomes in safety, efficiency, and effectiveness.
In this prospective, single-arm, multicenter U.S. Food and Drug Administration (FDA) pivotal study, 339 patients with persistent AF across 43 sites received pulmonary vein isolation (PVI) and posterior wall isolation (PWI) with a pentaspline PFA catheter (Farawave, from Boston Scientific). Follow-up included 24-hour Holter monitoring at six months and 12 months. The primary safety endpoint was the incidence of predefined adverse events. The primary effectiveness endpoint was acute success and one-year freedom from atrial arrhythmia recurrence (>30 seconds), re-do ablation, electrical cardioversion, or escalation of antiarrhythmic drugs.
In the study, primary safety was 2.3 percent, including one pericarditis, one coronary spasm, and four pulmonary edema. Primary effectiveness was 63.5 percent at one year, with 8.5 percent of patients having a single, isolated AF recurrence. Freedom from symptomatic AF was 85.3 percent; efficacy varied based on operator experience. Overall, recurrences were short, with many not resulting in changes to therapy, the study found.
“These results are significant because they provide the data that will be used to help gain regulatory approval of this PFA technology for the treatment of persistent AF,” Dr. Reddy says.
Dr. Reddy is a pioneer in the study and practice of the approach, which offers enhanced precision and speed compared to traditional ablation methods, while significantly reducing risks such as esophageal damage, pulmonary vein injury, and nerve damage. He has studied the PFA system for more than 10 years, and was lead author of the ADVENT clinical trial, which paved the way for approval of the PFA system by the FDA in January 2024. “Our study confirmed the procedure is a safe and effective option for treating paroxysmal AF, combining significantly shorter ablation times and a quicker learning curve for physicians,” Dr. Reddy says. He and his team achieved a major milestone in February 2024 by performing a groundbreaking procedure utilizing PFA technology, soon after it received FDA approval.
The Farawave pulsed field ablation catheter.
For decades, thermal energy for catheter ablation has been the accepted standard in cardiac electrophysiology for treating AF. The downside to thermal energy sources—including radiofrequency, cryotherapy, laser, and ultrasound—is the indiscriminate destruction of all tissue types. As a result, the procedure continues to be associated with infrequent but potentially serious complications such as pulmonary vein stenosis, stroke, phrenic nerve palsy, and atrio-esophageal fistula.
PFA instead treats symptomatic paroxysmal AF through a more tissue-preferential form of energy delivery that is both safer for patients and relatively easy for operators to learn and use. Positive 12-month data from the pivotal ADVENT trial, the first randomized study to directly compare the efficacy and safety of a PFA system against standard-of-care ablation, showed a success rate of 73.3 percent for PFA, comparable to the 71.3 percent seen in the thermal ablation group. The study included nearly 700 patients with paroxysmal AF at 30 facilities in the United States. All patients were treated with the FARAPULSE™ PFA System from Boston Scientific, which had earned the FDA’s breakthrough device designation in 2019. The results, reported in November 2023 in The New England Journal of Medicine, met the study’s predetermined criteria for noninferiority.
Another large-scale study led by Mount Sinai found that among patients who underwent PFA for paroxysmal or persistent AF, there was no significant difference between men and women in one-year freedom from recurrent atrial arrhythmia or major adverse events. That study was published in October 2023 in JAMA Cardiology.
PFA, characterized by its application of more controlled high-intensity pulses over very short duration, is now poised to become the field’s next major advance, Dr. Reddy says. “The system features a catheter with 122 gold electrodes, each capable of mapping the patient’s cardiac anatomy and electrical activity,” he explains, “while delivering PFA energy to treat atrial fibrillation.” The catheter uses contact sensing to determine which electrodes are in contact with cardiac tissue to help ensure therapy is delivered effectively to the heart. During the procedure, adjacent myocardial cell membranes are destabilized, resulting in nanoscale pores, increased cell membrane permeability, and leakage of cell contents. This phenomenon triggers either immediate necrosis or delayed apoptotic cell death.
“PFA makes it easy to quickly position the ablation catheter at each pulmonary venous origin, then ensure that the electrodes are in good contact with the tissue to safely and rapidly isolate the vein by delivering pulsed field energy,” adds Dr. Reddy, one of the world’s leading cardiac electrophysiologists. “For these reasons and more, I believe that pulsed field ablation will become the workhorse of our field, and that its adoption will be quick and extensive.”
Dr. Reddy serves as an unpaid consultant to Boston Scientific, the manufacturer of the PFA catheter technology studied in the MANIFEST-17K study and the Advantage AF trial.
An interview with the Journal of the American College of Cardiology on the ADVENT trial.
Featured

Vivek Reddy, MD
Director of Cardiac Electrophysiology, and the Leona and Harry B. Helmsley Charitable Trust Professor of Cardiac Electrophysiology