A small interfering RNA (siRNA) investigational therapy that inhibits a gene involved in lipoprotein metabolism was shown to significantly reduce the levels of cholesterol and triglycerides in individuals with mixed hyperlipidemia, in a global clinical trial led by Robert Rosenson, MD, Professor of Medicine (Cardiology) at the Icahn School of Medicine at Mount Sinai, and Director of Lipids and Metabolism for the Mount Sinai Health System.
In addition to seeing promising preliminary results related to safety and efficacy, the Mount Sinai researchers found RNA interference-based therapy zodasiran to be a potential option for substantially reducing a number of atherogenic lipoproteins while requiring less frequent dosing than conventional therapies. The results were presented as a late-breaking clinical trial at the European Atherosclerosis Society Congress in May 2024 in Lyon, France, and simultaneously published in The New England Journal of Medicine.
“Our study represents one of the first trials of an RNA inhibitor of ANGPTL3 with advantages like durable gene silencing and infrequent dosing,” says Dr. Rosenson, the lead author. “For patients with mixed hyperlipidemia and persistent elevations in LDL cholesterol, non-HDL cholesterol and remnant cholesterol, zodasiran could expand the opportunities for lowering multiple ‘bad’ cholesterol containing lipoproteins, beyond conventional therapies such as statins, potentially leading to more favorable outcomes for patients.”
Zodasiran, made by Arrowhead Pharmaceuticals, targets a specific gene expressed in hepatocytes known as angiopoietin-like protein 3 (ANGPTL3), which plays a role in regulating levels of low-density lipoprotein (LDL), non-HDL cholesterol (a measure of all the “bad” cholesterol in the blood, including LDL), and remnant cholesterol carried in triglyceride-containing lipoproteins. Various research has identified these components as increasing risk of atherosclerotic cardiovascular disease.
“Our study represents one of the first trials of an RNA inhibitor of ANGPTL3 with advantages like durable gene silencing and infrequent dosing.”
Robert Rosenson, MD
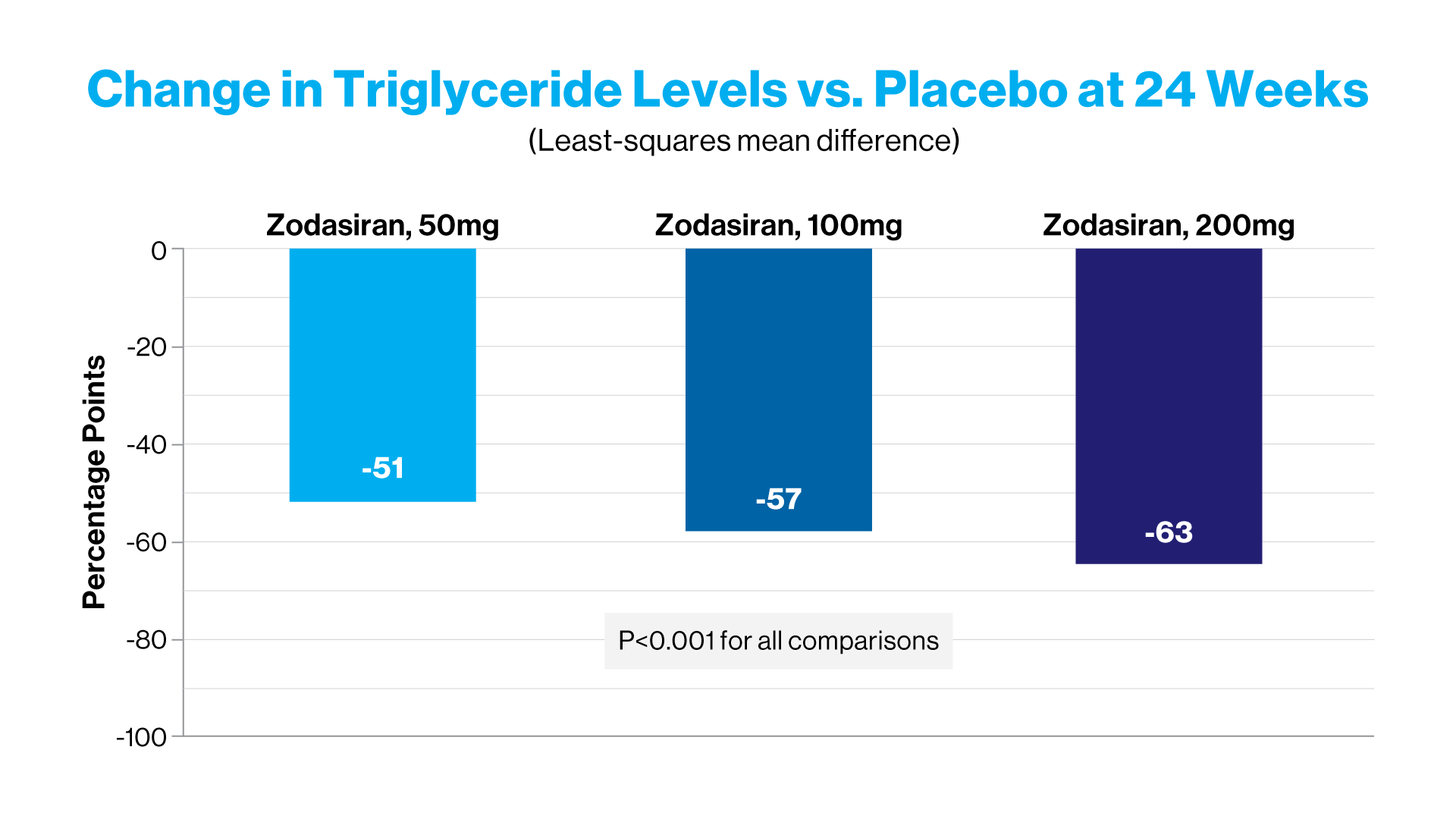
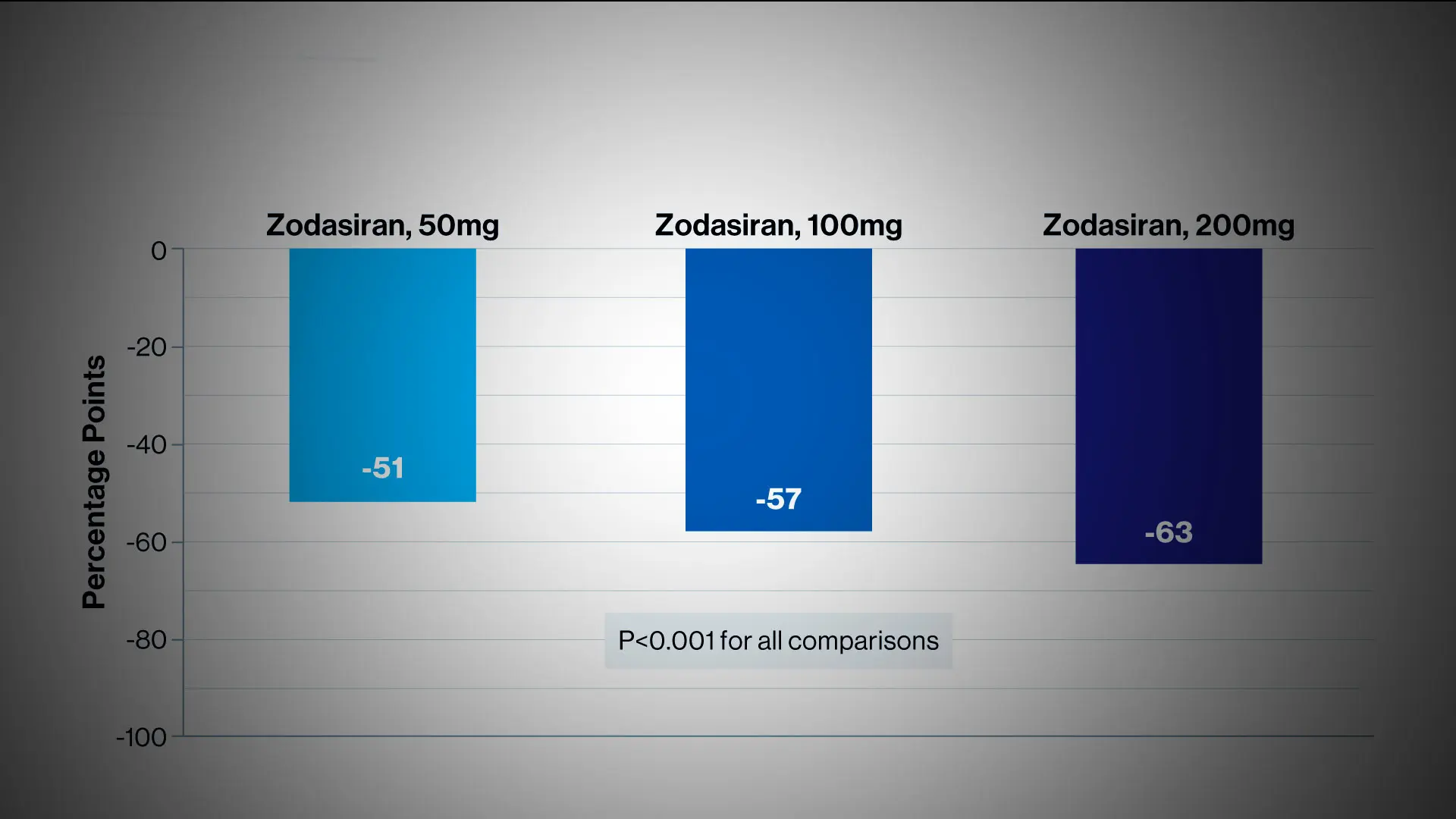
At week 24, zodasiran was associated with significant decreases in fasting triglyceride levels, as compared with placebo. And each increasing dose of zodasiran was associated with a greater reduction in the triglyceride level.
The double-blind, placebo-controlled, phase 2b trial, known as ARCHES-2, was conducted at 25 sites across four countries and funded by Arrowhead. It evaluated the safety and efficacy of zodasiran in adults with mixed hyperlipidemia—with fasting triglyceride level of 150 to 499 mg per deciliter and either an LDL cholesterol level of ≥70 mg per deciliter or a non-HDL cholesterol level of ≥100 mg per deciliter. Patients were randomly assigned to receive subcutaneous injections of zodasiran (50, 100, or 200 mg) or placebo on day 1 and week 12. The primary endpoint was the percent change in the triglyceride level from baseline to week 24.
In the 204 participants, who also received a background therapy of standard-of-care medications including statins, the researchers observed substantial reductions in all lipid level parameters monitored. These included lowering triglycerides by 54 to 74 percent compared to placebo, LDL cholesterol by up to 20 percent, non-HDL cholesterol by up to 36 percent, and remnant cholesterol by 73 to 82 percent. Remnant cholesterol measures the amount of “leftover” very-low-density lipoprotein (VLDL) particles. In patients with triglycerides above 880 mg/dL, remnant cholesterol is found in chylomicron as well as VLDL particles. It is measured directly or by an estimation that requires adding HDL and directly measured LDL and subtracting that sum from the individual’s total cholesterol.
The reduction in remnant cholesterol is particularly important because those remnants may contain up to four times more cholesterol per particle than LDL. Moreover, prior research has demonstrated an association between elevated remnant cholesterol and increased risk of cardiovascular disease. The Mount Sinai researchers suggested that, based on prior genetic studies, the magnitude of remnant cholesterol reduction evidenced by zodasiran in their study could translate into a 20 percent decrease in recurrent major cardiac events.
The ARCHES-2 study also found zodasiran effective in lowering apolipoprotein B, a lipid-transporting protein in the body that has been linked to increased risk of heart disease at high levels. “In contrast to fibrates and fish oils, zodasiran lowers apolipoprotein B and thus may be a more promising potential therapy for reducing the risk of cardiovascular events,” Dr. Rosenson says.
The findings from this study in patients with mixed hyperlipidemia build on prior efforts to modulate ANGPTL3 with evinacumab, the fully human monoclonal antibody against the ANGPTL3 protein, approved by the U.S. Food and Drug Administration to treat patients with homozygous familial hypercholesterolemia. “It’s our contention,” Dr. Rosenson says, “that based on these promising results, further studies are warranted to determine the potential of zodasiran, an investigational drug, to reduce the risk of cardiovascular events in a broad range of patients through a single therapy that targets all the lipoprotein fractions.”
Robert Rosenson, MD, reports grant/research support from (all paid to institution, not individual): Amgen, Arrowhead, Novartis, Eli Lilly, Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Editas, Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, Verve; non-promotional speaking fee from Meda Pharma; royalties from Wolters Kluwer, stock shareholder in MediMergent, LLC (significant).
Featured

Robert Rosenson, MD
Professor of Medicine (Cardiology), and Director of Cardiometabolic Disorders