Mount Sinai researchers identified a key driver of fibromuscular dysplasia (FMD), a poorly understood disorder that can lead to high blood pressure, heart attack, or stroke. In a study published in September 2024 in Nature Cardiovascular Research, the team said changes in the gene UBR4 played an important role as a key driver of FMD, and said the discovery suggests a potential therapeutic avenue for this challenging disease.
“Although fibromuscular dysplasia was first recognized more than 80 years ago, until now very little was known about its causes, pathobiology, or possible treatment,” says Jason C. Kovacic, MD, PhD, Professor of Medicine (Cardiology) at the Icahn School of Medicine at Mount Sinai and senior author of the study. “By creating the first mouse model, we gained critical insights into the processes that trigger FMD, including the role of the protein coding gene UBR4 and its associated gene expression supernetwork, which regulates vascular function in the body.”
The pathobiology of FMD—which affects approximately 5 percent of women— involves arterial lesions of stenosis, dissection, tortuosity, ectasia, and aneurysm, which can lead to hypertension, stroke, myocardial infarction, and even death. Until Mount Sinai’s systems biology study, known as DEFINE-FMD, there were no animal models for FMD and few insights into the mechanisms underlying its pathobiology.
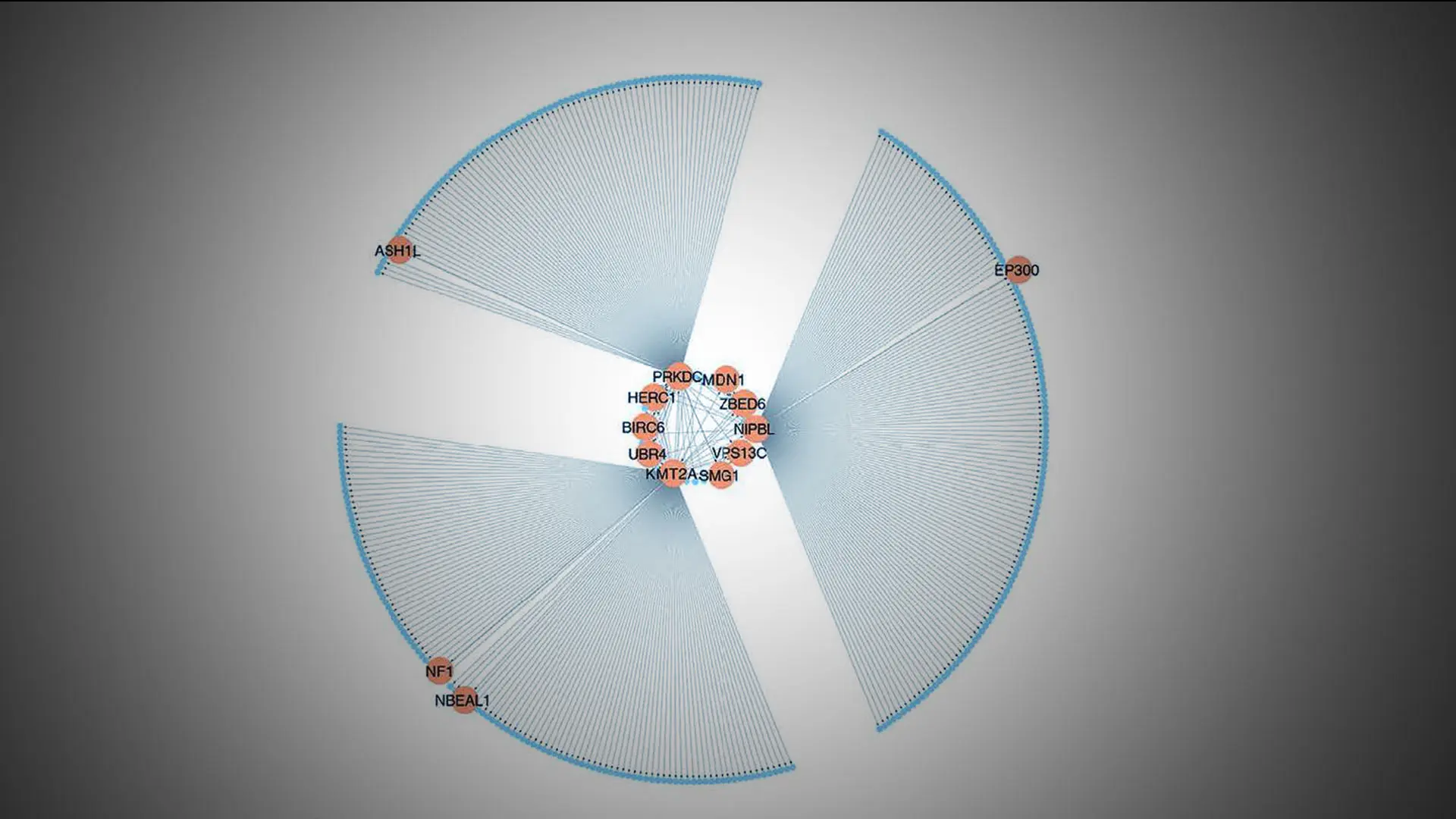
In the study, by integrating DNA genotype and RNA sequence data from primary fibroblasts of 83 patients with FMD and 71 matched healthy controls, the team inferred 18 gene regulatory co-expression networks, four of which were found to act together as an FMD-associated supernetwork. Additional analyses identified UBR4 (ubiquitin protein ligase E3 component n-recognin 4) as an important key driver gene of this supernetwork, which also showed high expression levels in arterial tissues, prompting further investigation. In a series of in vitro and in vivo (mouse) experiments, the team investigated the role of UBR4 as a driver of FMD. The team found that UBR4 knockdown or knockout had a major effect on the expression levels of other genes in the supernetwork. By selectively knocking out the UBR4 gene in smooth muscle cells in mice, and thus the activity of the supernetwork, the team was able to create the first animal model for FMD and provide key insights into the processes that cause this disease.
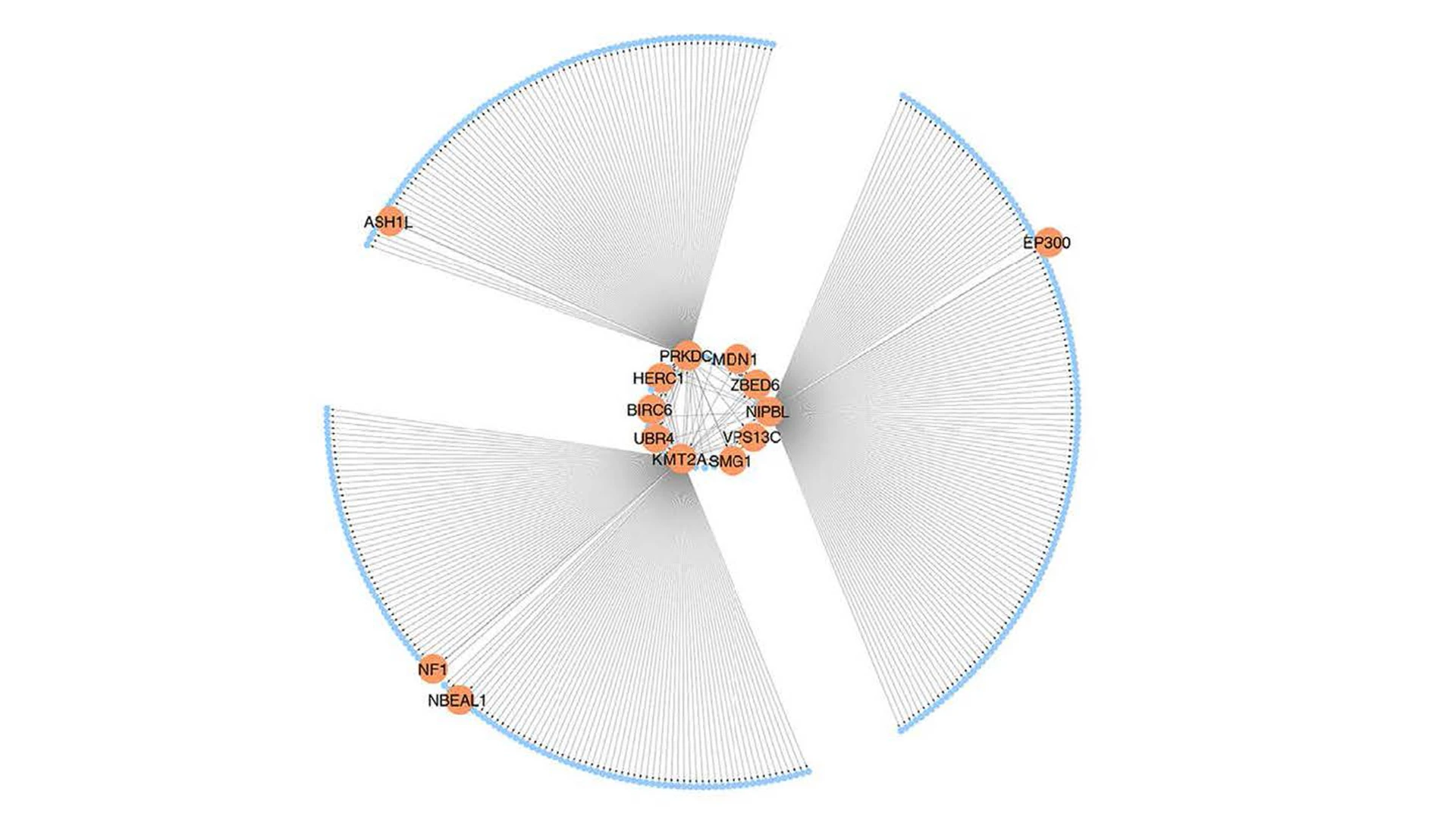
A visual representation of the SN-A, an important gene regulatory co-expression supernetwork governing fibromuscular dysplasia.
“These insights included the finding that changes in UBR4 levels—which cause significant changes in the expression levels of other genes in the FMD-associated supernetwork—collectively led to major changes in vascular cell function,” says co-author Jeffrey W. Olin, DO, Professor of Medicine (Cardiology) at the Icahn School of Medicine at Mount Sinai and an internationally known expert in vascular medicine. “These alterations in turn led to a demonstrable widening of the arteries in mice, which is one of the features of FMD in humans.”
The study has some limitations, the team points out. FMD has a complicated phenotype in humans, and it is rare that a given patient will manifest all features of the disease (arterial “string of beads”, stenosis, tortuosity, dissection, ectasia, aneurysm, and occlusion). The mouse models developed in this study did not exhibit all FMD features, but tended to exhibit prominent arterial dilation. It remains to be determined whether future FMD mouse models could exhibit these other features of FMD.
However, by identifying a gene and gene regulatory network that appear to account for a significant portion of FMD heritability, scientists believe they have taken a major step toward a therapeutic solution. "Our study opens the door to targeted modulation of UBR4 and its disease-relevant gene regulatory network, and that could hold tremendous promise for the many people, particularly women, with this condition,” Dr. Kovacic says. “These exciting findings are encouraging us to continue our work with colleagues around the world to shed further light on a disease that until now was largely a blank slate.”
Funding for this study was supported by grants from the National Heart, Lung, and Blood Institute at the National Institutes of Health and additional philanthropic support.
Featured

Jeffrey W. Olin, DO
Professor of Medicine (Cardiology)

Jason C. Kovacic, PhD
Professor of Medicine (Cardiology)