Mount Sinai is advancing the understanding of the effects of psychosocial stress on cardiovascular disease with research supported by renewal of an $11.5 million award from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
"To address residual cardiovascular risk in patients, our research program aims to bolster our mechanistic understanding of how psychosocial stress impacts the immune system and inflammatory atherosclerosis," says Principal Investigator Zahi Fayad, PhD, Director of the Biomedical Engineering and Imaging Institute, the Lucy G. Moses Professor in Medical Imaging and Bioengineering, and Professor of Medicine (Cardiology) at the Icahn School of Medicine at Mount Sinai.
In the initial five-year grant, the team significantly advanced knowledge on psychosocial stress and cardiovascular disease by identifying connections between the nervous, immune, and vascular systems, Dr. Fayad says.
“The research supported by this grant is both fundamental and translational. On the one hand, the studies are investigating molecular and cellular mechanisms through which stress perception affects health and disease. On the other hand, the research is exploring how clinical manifestations of stress modulate inflammation in people,” says Filip K. Swirski, PhD, Director of Mount Sinai’s Cardiovascular Research Institute, and an investigator in the research. “It’s a comprehensive approach that has already revealed important insights on the nature of stress.”
One pivotal study led by the group, published in The Lancet in 2017, revealed that individuals with higher activity in the amygdala (a region of the brain involved in stress and fear response) showed an increased risk of cardiovascular events such as heart attacks or strokes. This finding underscores the heart-brain connection, highlighting how emotional stress can manifest physically in the body, contributing to inflammation and atherosclerosis. “The pathway identified involves emotional stress leading to increased white blood cell production and inflammation, which in turn accelerates atherosclerosis,” Dr. Fayad says. “The process was previously demonstrated in animal models but was confirmed in humans through this work.”
A study published in 2022 in Nature reported insights into the mechanistic pathways linking stress networks in the brain to peripheral leukocytes, showing that distinct brain regions shape leukocyte distribution and function throughout the body during acute stress in mice. The study showed that stress perception mechanisms influence atherosclerosis development, using advanced imaging tools the team developed to study the brain regions related to the perception of stress.
“Collaboratively, we have generated innovative research questions we’re now eager to explore.”
Zahi Fayad, PhD
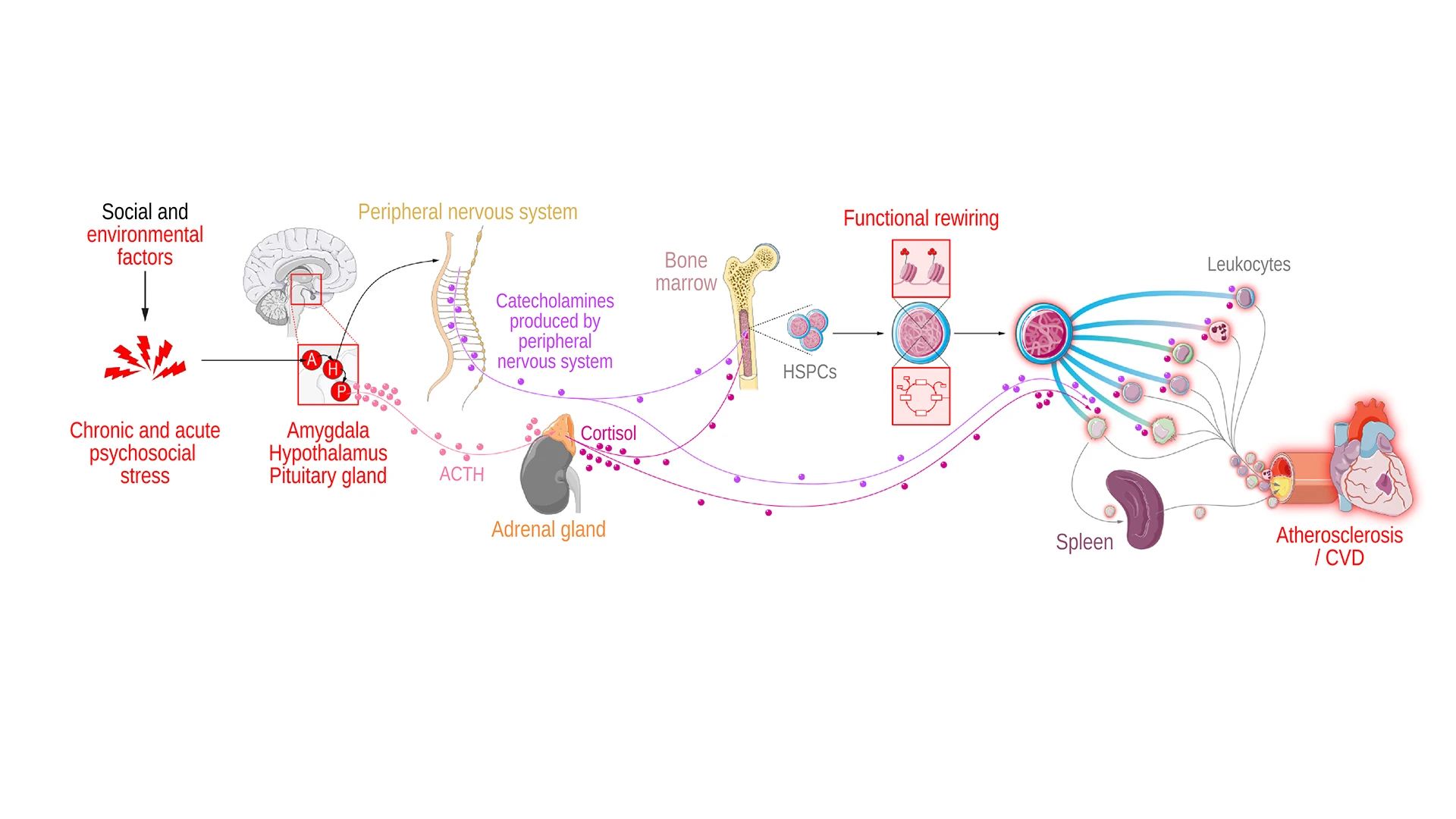
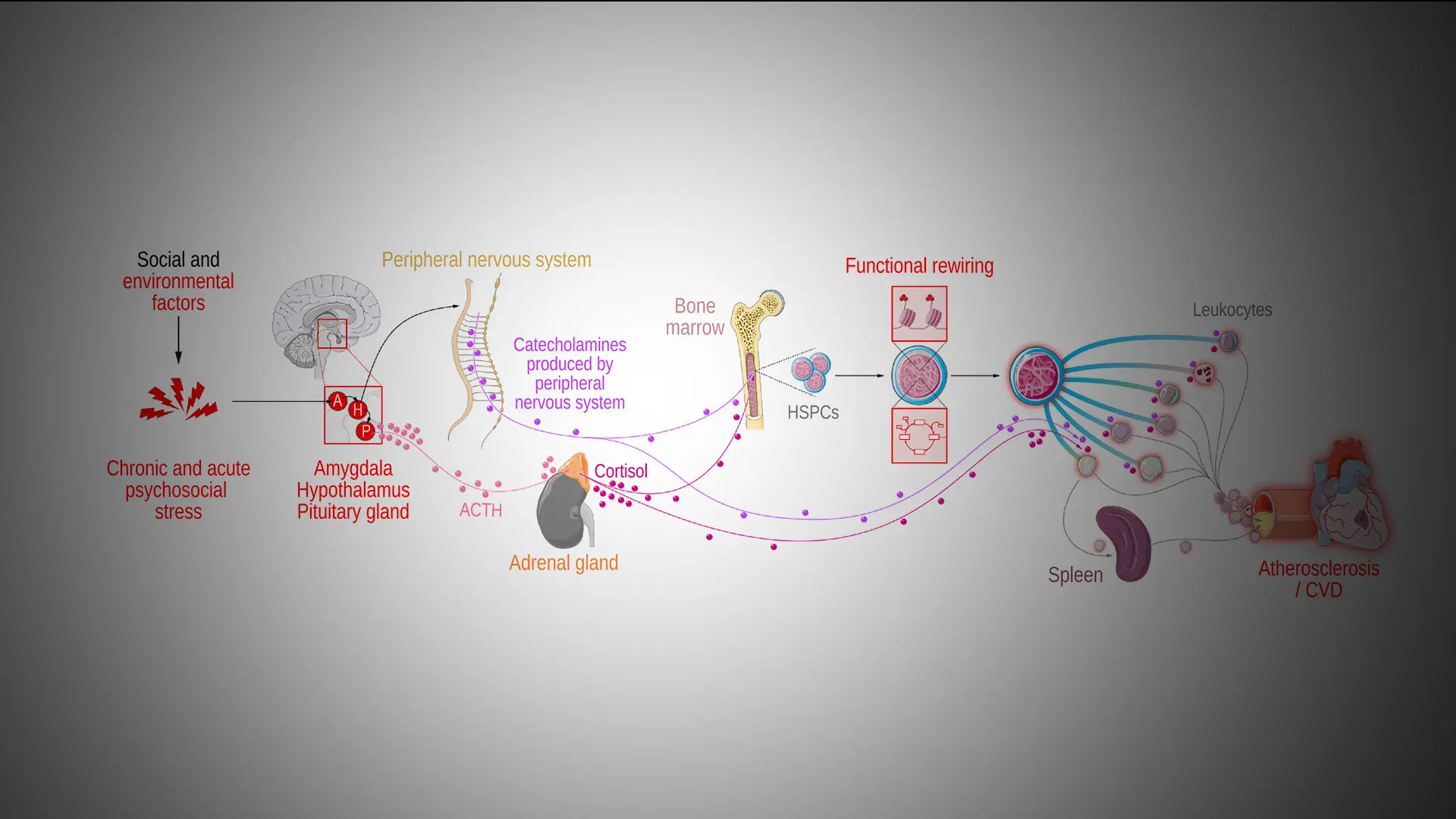
Chronic stress influences many brain regions, including the hypothalamus and amygdala. Increased hypothalamus activity affects the pituitary gland, which induces adrenal cortisol production. Following this, sympathetic nervous system activation results in catecholamine production, which along with corticosteroids and catecholamines has a direct effect on leukocyte function. Finally, immune cell reprogramming affects atherosclerotic plaque development and regression.
In an earlier effort, the team made substantial progress in translational imaging studies to measure stress-associated neurobiology as it relates to cardiovascular disease. In a significant study, published in 2019 in the Journal of the American College of Cardiology, researchers studied a database of 509 individuals, median age 55, who underwent clinically indicated whole-body positron emission tomography and met criteria including absence of known cardiovascular disease or active cancer. The study found that among these participants, lower social economic status (SES) was associated with higher amygdalar activity; and that it independently predicted major adverse cardiovascular events via a pathway that includes higher amygdalar activity, bone marrow activity, and arterial inflammation. “These findings illuminate a stress-associated neurobiological mechanism by which SES disparities may potentiate adverse health outcomes,” the study said.
“Collaboratively, we have generated innovative research questions we’re now eager to explore,” Dr. Fayad says. Building on the previous five-year grant, the research team is broadening their perspective to better understand immune reprogramming in atherosclerotic disease aggravated by psychosocial stress. The work will involve:
Evaluating various brain circuits
Utilizing diverse imaging methods
Conducting in-depth analyses (omics) for optimal integration and harmonization of data
Dr. Fayad is leading a multidisciplinary team from Icahn Mount Sinai, Massachusetts General Hospital, New York University, and Radboud University Medical Center in the Netherlands to complete the interrelated experiments. At Icahn Mount Sinai, the team includes Dr. Swirski, Scott Russo, PhD, Philip Robson, PhD, Li Shen, PhD, Abraham Teunissen, PhD, MSc, BASc, Maria Giovanna Trivieri, MD, PhD, and Mandy van Leent, MD, PhD.
"This research has the potential to not only advance our scientific understanding of neural control of immune mechanisms that drive cardiovascular diseases, but also could offer approaches to managing such diseases in individuals experiencing prolonged psychosocial stress, including identifying novel therapeutic targets, treatments, and preventive measures," says Eric J. Nestler, MD, PhD, Nash Family Professor of Neuroscience, Director of The Friedman Brain Institute, and Dean for Academic Affairs at Icahn Mount Sinai, and Chief Scientific Officer of the Mount Sinai Health System.
Featured

Zahi Fayad, PhD
Lucy G. Moses Professor in Medical Imaging and Bioengineering, and Professor of Medicine (Cardiology)

Filip K. Swirski, PhD
Arthur and Janet C. Ross Professor of Medicine (Cardiology); Professor of Diagnostic, Molecular, and Interventional Radiology; Director of The Cardiovascular Research Institute