The Mount Sinai Hospital’s Cardiac Catheterization Laboratory pioneered two innovative procedures in 2023, continuing its leadership in the field. The first involved employing the world’s smallest heart pump system to provide temporary mechanical circulatory support during high-risk percutaneous coronary intervention (PCI), while the second procedure treated a patient’s uncontrolled high blood pressure with the groundbreaking Symplicity Spyral renal denervation (RDN) system.
In November 2023, a surgical team at Mount Sinai Fuster Heart Hospital became the first in the Northeast to undertake the one-hour, minimally invasive Symplicity Spyral RDN procedure, newly approved by the U.S. Food and Drug Administration. “This system could be a game changer for untold numbers of patients who can’t get their blood pressure under control through antihypertensive medication and lifestyle changes,” says Samin K. Sharma, MD, Director of Interventional Cardiology for the Mount Sinai Health System, who was a leader of the surgical team. “We believe this technology could be a major step toward reducing heart attacks, strokes, and other debilitating cardiac events.”
Developed by Medtronic, the Symplicity Spyral system lowers blood pressure by delivering radiofrequency energy through the wall of the renal artery to denervate the kidney from sympathetic nerve hyperactivity. During the procedure, a thin catheter is inserted into the artery leading to the kidneys. Once it is in place, the surgeon administers energy through the system to calm excessive activity of the nerves surrounding the renal arteries. At the conclusion of the adjunctive treatment—which was proven safe and effective in several clinical trials—the catheter is removed.
During the procedure, the Mount Sinai team treated a 45-year-old man who had attempted to manage his hypertension with three medications. The team included Dr. Sharma; Annapoorna S. Kini, MD, Director of the Cardiac Catheterization Laboratory; Prakash Krishnan, MD, Director of Endovascular Interventions at the Cath Lab; and George Dangas, MD, PhD, Director of Cardiovascular Innovation at the Zena and Michael A. Wiener Cardiovascular Institute. After a short observation period following surgery, the patient returned home with his blood pressure within the normal range.
In June 2023, Dr. Sharma also performed the first U.S. procedure using the miniaturized Elevate percutaneous left ventricular assist device (pLVAD), designed to provide cardiologists with a powerful new tool to support high-risk patients during complex procedures. Mount Sinai was the top enroller for the heart pump system’s FDA-approved early feasibility study, which announced its completion in September 2023 after successfully treating 15 patients at The Mount Sinai Hospital and two other centers. That study is the first step in a clinical program by the device’s developer, Magenta Medical, to secure approval of the heart pump in the United States.
“We believe this technology could be a major step toward reducing heart attacks, strokes, and other debilitating cardiac events.”
Samin K. Sharma, MD

The Symplicity Spyral system, including this device, lowers blood pressure by delivering radiofrequency energy through the wall of the renal artery to denervate the kidney from sympathetic nerve hyperactivity.
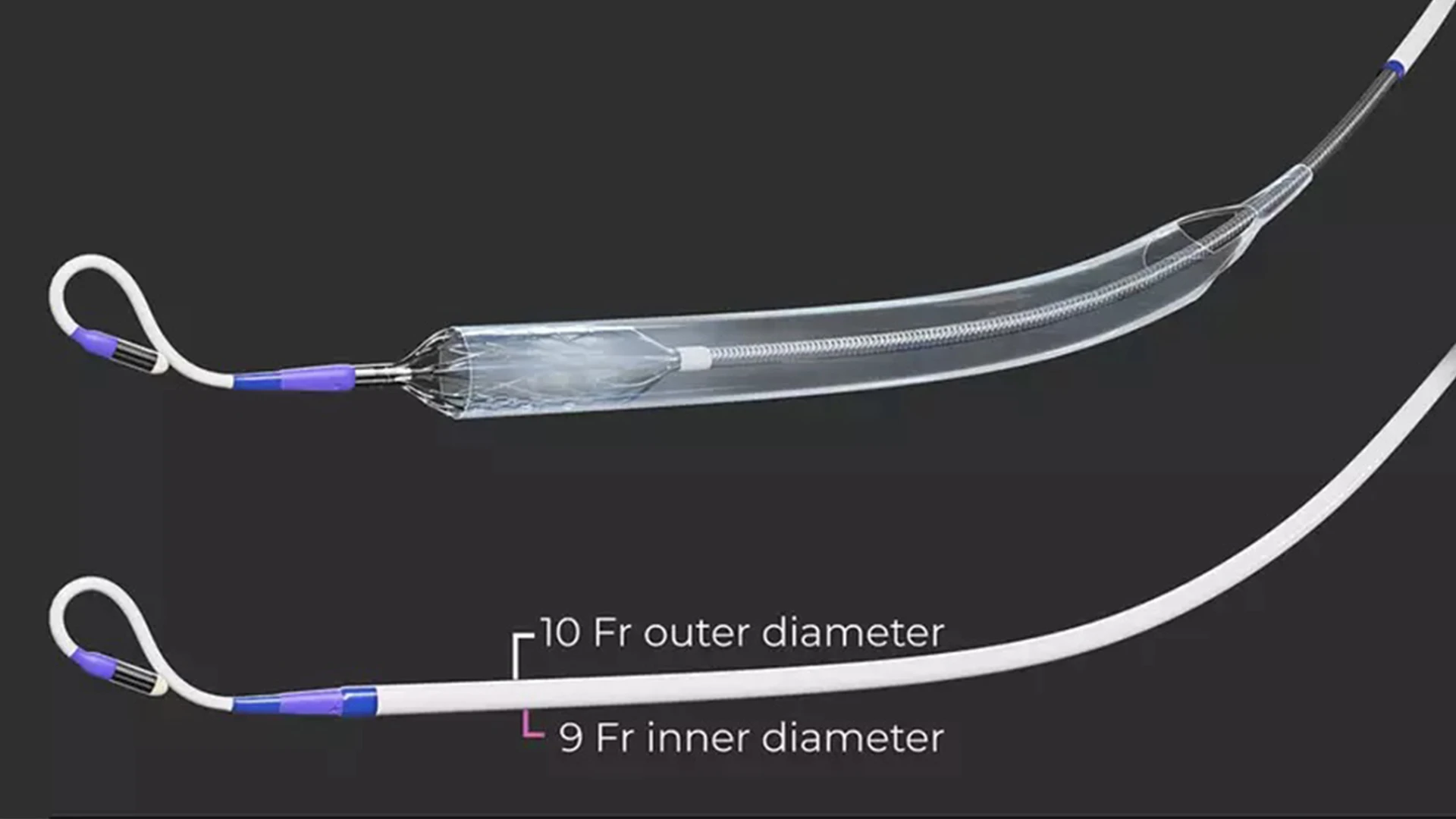
The miniaturized Elevate percutaneous left ventricular assist device provides cardiologists with a new tool to support high-risk patients during complex procedures.

The team performing the Symplicity Spyral procedure included Prakash Krishnan, MD, Samin K. Sharma, MD, Annapoorna S. Kini, MD, George Dangas, MD, PhD, Deepak L. Bhatt, MD, MPH, and staff of The Mount Sinai Hospital Cardiac Catheterization Laboratory.
The investigational device miniaturizes a powerful blood pump to fit an 8 French (Fr) delivery system, the smallest crimping profile of any such device. The Elevate heart pump is inserted over a guidewire through commercially available 10 Fr introducer sheaths, which require a small puncture in the groin. The flow of the pump is adjusted based on the clinical characteristics of the patient, with the ability to surpass 5 L/min of mean flow, making it the most powerful pump of its kind.
“Having used Elevate in nine complex, high-risk PCI procedures, I can truly appreciate its advantages,” notes Dr. Sharma, who has consistently recorded among the highest PCI success rates and lowest mortality rates among interventional cardiologists in New York State. He cites three specific benefits over existing mechanical circulatory support systems: small insertion profile, ease of use, and high pump flow. “These features make it possible to smoothly navigate the device even through hostile vascular environments, which is no small advantage since atherosclerosis affects the entire arterial tree.”
Innovation, safety, and high volume are hallmarks of The Mount Sinai Hospital’s Cardiac Catheterization Laboratory. For the 24th consecutive year, in 2023 the lab and an interventionalist received the highest two-star safety rating from the New York State Department of Health (NYSDOH) for PCI—one of the most common procedures for patients with coronary artery disease.
In a highlight of the NYSDOH report, Dr. Kini received the two-star rating for significantly lower 30-day risk-adjusted mortality for PCI in all cases and in nonemergency cases. She was the only interventionalist in the state to receive this rating in both categories, while performing 2,844 procedures in the latest period reported, December 1, 2016, to November 30, 2019.
During the three-year period, The Mount Sinai Hospital had a risk-adjusted PCI mortality rate of 0.85 percent for all of its cases—emergency and nonemergency—significantly lower than the statewide average of 1.22 percent, while performing the largest number of procedures (10,347). For nonemergency cases, Mount Sinai’s PCI mortality rate was 0.50 percent, compared with the statewide average of 0.79 percent.
“Despite taking on some of the most challenging referrals, our Cath Lab has received the double-star rating again,” says Dr. Kini, the Zena and Michael A. Wiener Professor of Medicine (Cardiology). “I believe that our efforts as educators and investigators—in our conferences, live cases, publications, educational applications, and clinical trials—bring us to the forefront of the field.”
Featured

Annapoorna S. Kini, MD
Director of the Cardiac Catheterization Laboratory at The Mount Sinai Hospital, and the Zena and Michael A. Wiener Professor of Medicine

Samin K. Sharma, MD
Director of Interventional Cardiology, The Mount Sinai Hospital, and Anandi Lal Sharma Professor of Medicine in Cardiology
