Patients with severe hypercholesterolemia who do not achieve their low-density lipoprotein cholesterol (LDL-C) thresholds, despite treatment with maximally tolerated combinations of lipid-lowering therapies, have an increased risk of atherosclerotic cardiovascular disease. For such patients, a therapeutic pathway targeting angiopoietin-like protein 3 (ANGPTL3) shows promise and is the subject of a series of trials led by Robert S. Rosenson, MD, Professor of Medicine (Cardiology), and Director of Cardiometabolic Disorders.
The aim is to widen the scope of therapies such as evinacumab, which was approved by the U.S. Food and Drug Administration in 2021 as an add-on treatment for patients aged 12 years and older with homozygous familial hypercholesterolemia, a relatively rare genetic condition that causes severely high cholesterol, and high rates of cardiovascular events, even in children.
Genetic studies have shown that people who are missing or have low levels of ANGPTL3 are known to have very low lifelong levels of LDL cholesterol and rarely suffer from atherosclerotic cardiovascular disease, Dr. Rosenson says. The therapy evinacumab is a fully human monoclonal antibody that inhibits ANGPTL3 and lowers LDL cholesterol through an LDL receptor independent pathway.
A study led by Dr. Rosenson and published in September 2023 in JAMA Cardiology evaluated the longer-term efficacy and safety of evinacumab in patients with refractory hypercholesterolemia. It found that the therapy provided sustained reductions in LDL-C level and was well tolerated.
The phase 2 randomized clinical study included double-blind treatment periods, in which patients received either evinacumab or a placebo—16 weeks for patients receiving subcutaneous treatment or 24 weeks for those receiving intravenous therapy. This was followed by a 48-week open-label titration period for the intravenous group, and a 24-week follow-up period. Patients were recruited from 85 sites across 20 countries, including some with primary hypercholesterolemia, defined as heterozygous familial hypercholesterolemia (HeFH) or established clinical atherosclerotic cardiovascular disease without familial hypercholesterolemia.
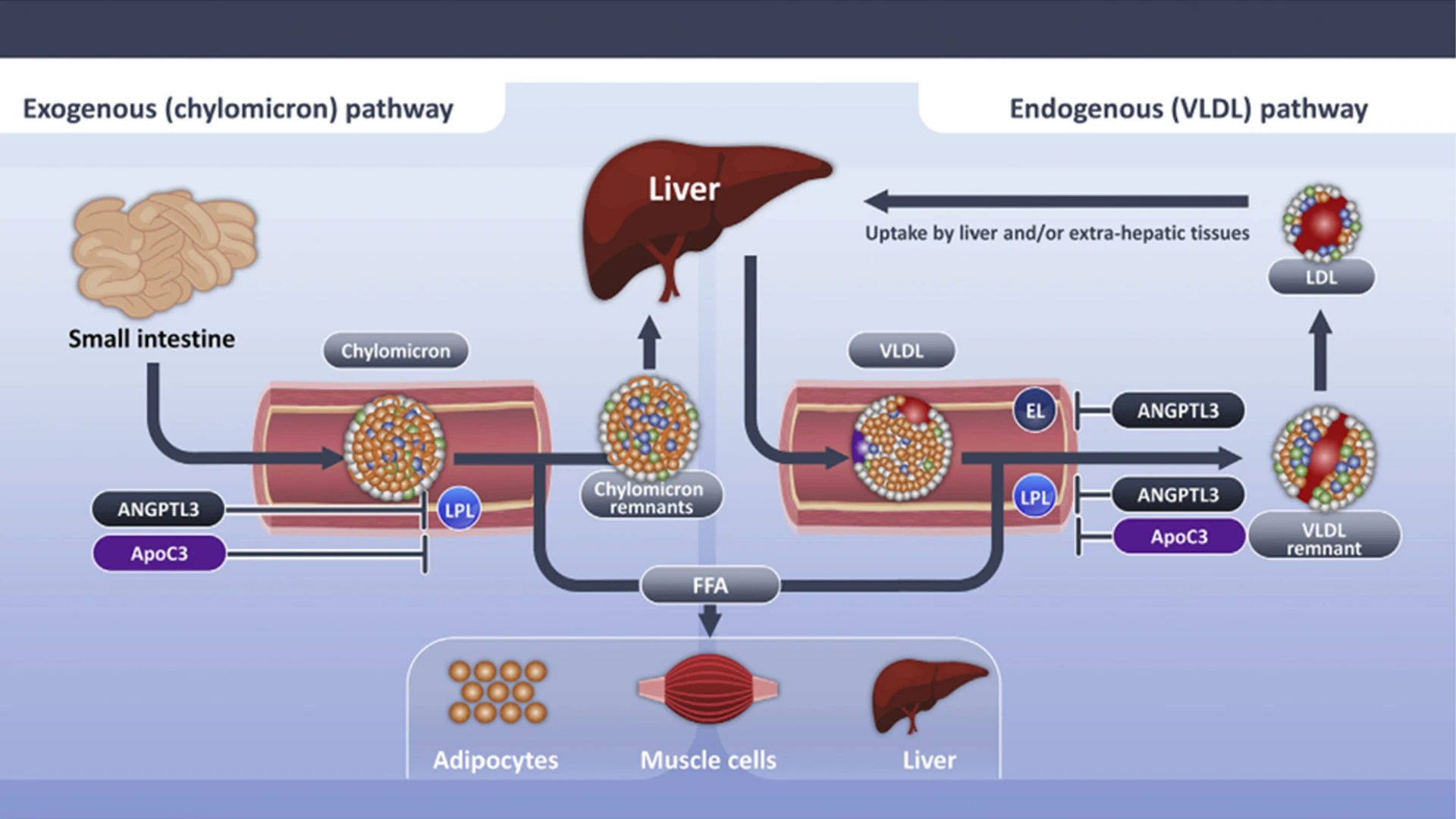
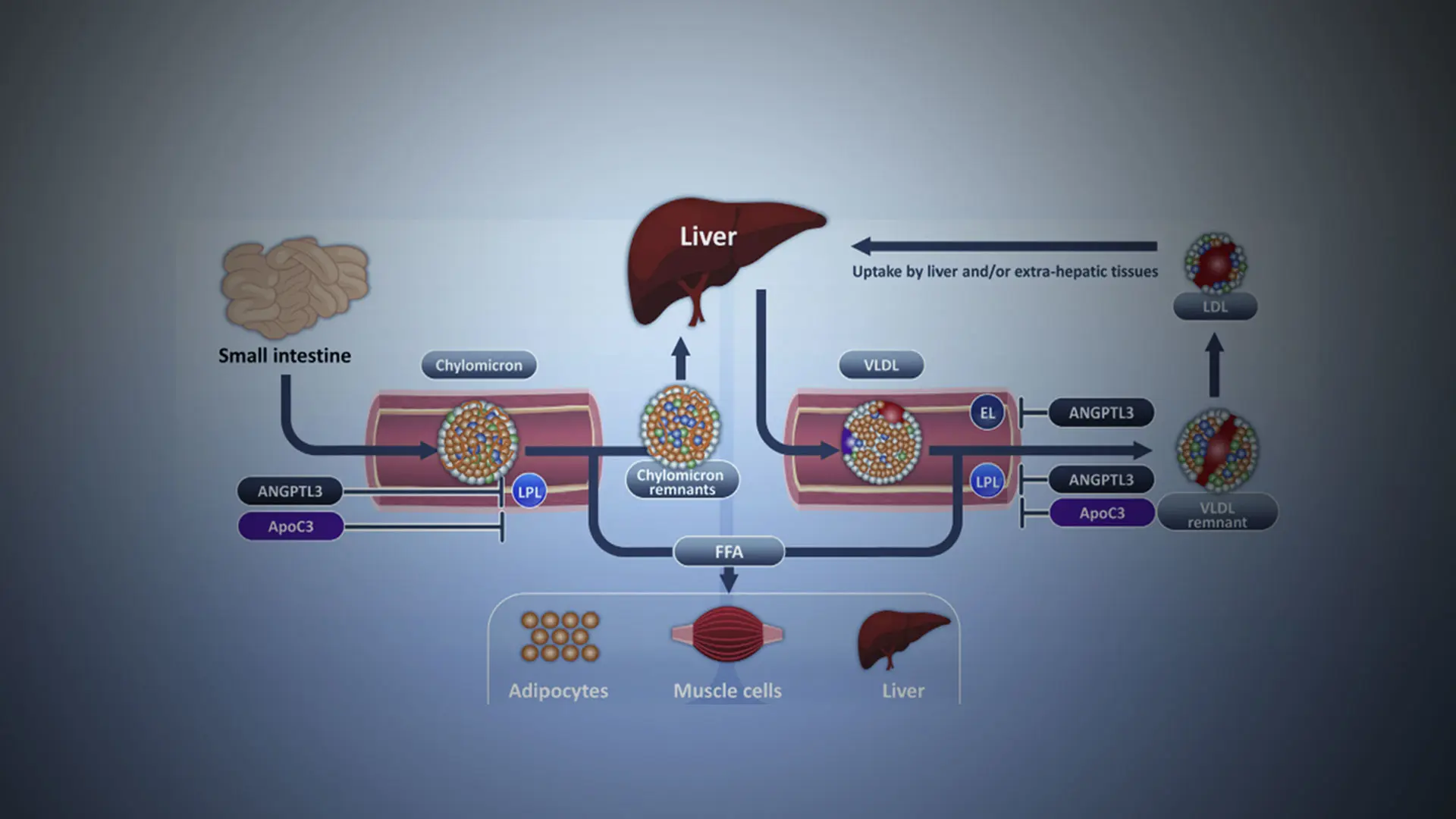
Triglyceride-rich lipoproteins are hydrolyzed by lipoprotein lipase, which elaborates free fatty acids that are used as an energy source by skeletal muscle or stored for future use in the liver or adipocytes. Lipoprotein lipase activity is inhibited by angiopoietin-like 3 protein and apolipoprotein C-III. Delayed clearance of triglyceride-rich lipoproteins fosters formation of a more cholesterol-enriched remnant particle.
A total of 96 patients with a mean age of 54.4 received evinacumab, 15 mg/kg intravenously every four weeks. Most of these patients (92 of 96) were taking a proprotein convertase subtilisin kexin 9 (PCSK9) inhibitor, 81 were receiving a statin, with 51 receiving a high-intensity statin (all patients were receiving maximally tolerated doses of statin), and 36 patients were taking ezetimibe, a drug that limits the absorption of cholesterol from the intestine.
At the start of the trial, the mean baseline LDL-C level was 145.9 mg/dL on the background of a PCSK9 monoclonal antibody inhibitor (alirocumab [Pralent] or evolocumab [Repatha]) and maximal tolerated dose of a statin. At week 72, the end of the open-label period, evinacumab reduced the mean LDL-C level from baseline by 45.5 percent in the overall cohort. The therapy also reduced mean apolipoprotein B, non-high density lipoprotein cholesterol, total cholesterol, and median fasting triglyceride levels at week 72. Serious adverse events occurred in 9 of 96 patients; all were considered unrelated to study treatment.
These results demonstrated that, in patients with and without HeFH and refractory hypercholesterolemia, evinacumab provided significant and sustained reductions in LDL-C level. Researchers noted that larger studies in a broader population would be needed to define clinical efficacy and provide more robust long-term safety data.
The research builds on a significant study of evinacumab that was published in August 2020 in The New England Journal of Medicine. The study, supported by Dr. Rosenson as the lead site in the United States and writing committee member, found that evinacumab lowered LDL cholesterol levels for homozygous familial hypercholesterolemia patients, who are typically highly resistant to traditional statins.
“Homozygous familial hypercholesterolemia results from two variants affecting LDL receptor function, and occurs in 1 in 300,000 to 1 in 600,000. In contrast, Individuals who develop heterozygous familial hypercholesterolemia, or one variant in the LDL receptor or pathway, are about 1 in 244 to 1 in 313 individuals worldwide,” Dr. Rosenson says. “In any clinical development program, you look at the extreme phenotypes, genotypes, the top of the pyramid, and then you expand to a broader population. By lowering triglyceride, LDL cholesterol, non-HDL cholesterol, and non-LDL proteins, we are able to impact much larger segments of the population.” Further studies will evaluate small interfering RNA inhibitors and gene editing in the ANGPTL3 inhibitor pathway.
In a related study published in March 2023 in Nature Medicine, Dr. Rosenson’s team found that ANGPTL3 inhibitor therapy was also effective in lowering triglycerides in patients with severe hypertriglyceridemia (sHTG) who had at least one prior episode of acute pancreatitis. sHTG is a well-established risk factor for recurrent episodes of acute pancreatitis.
ANGPTL3 inhibitor therapy, intravenously administering evinacumab, inhibits two important regulators of lipoprotein metabolism, reducing triglycerides by up to 70 percent in people with sHTG who had at least some lipoprotein lipase activity, the key enzyme in triglyceride metabolism. However, in one of the three groups of sHTG patients with genetic mutations resulting in no LPL activity—a rare condition known as familial chylomicronemia syndrome—evinacumab was ineffective in lowering triglycerides. In the other groups, the triglycerides were reduced by a median of 65 to 82 percent. In addition, many other triglyceride endpoints were favorably impacted by evinacumab.
“In many ways, we are encouraged by the results of the study,” says Dr. Rosenson, a physician-scientist who specializes in patients with complex lipoprotein disorders. “In patients with no residual lipoprotein lipase activity, we wouldn’t have expected evinacumab to be effective since there is no enzymatic activity present to disinhibit. But in the broader population of sHTG patients, those with multifactorial chylomicronemia syndrome, it worked profoundly well. Even when the primary endpoints were not met—in familial chylomicronemia syndrome patients—we observed favorable changes in lipid and lipoprotein levels along with safety benefits that warrant further evaluation of this class of inhibitors in larger trials.”
Focusing on non-LDL-focused treatments facilitates successful treatment of patients with familial hypercholesterolemia as well as those with severe hypertriglyceridemia and at least one prior episode of acute pancreatitis from experiencing recurrent episodes of acute pancreatitis. “These studies establish that modification of remnant cholesterol as a causal pathway can help us effectively treat atherosclerotic cardiovascular disease for a larger group of patients than previously thought,” says Dr. Rosenson.
Featured

Robert Rosenson, MD
Professor of Medicine (Cardiology), and Director of Cardiometabolic Disorders