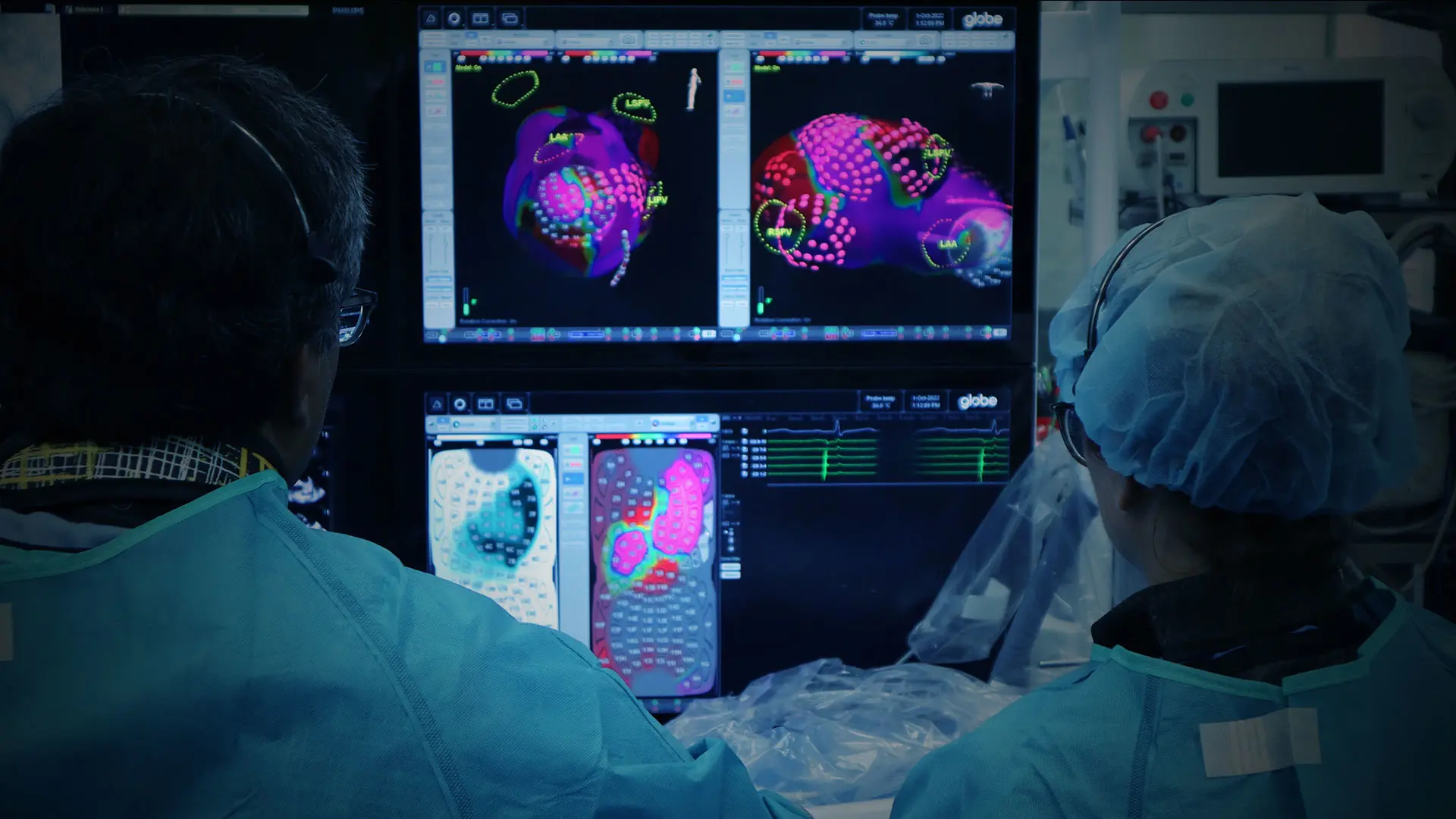
The first-in-human study of a next-generation system to treat atrial fibrillation (AF) using pulsed field ablation (PFA) therapy was conducted in 2022 by a team led by Vivek Reddy, MD, Director of Cardiac Arrhythmia Services, Icahn School of Medicine at Mount Sinai.
The team, co-led by Petr Neužil MD, in Prague, Czech Republic, successfully treated 38 patients, isolating 100 percent of the patients’ pulmonary veins using the Globe Pulsed Field System made by Kardium Inc.
The system features a catheter with 122 gold electrodes, each of which can map the patient’s cardiac anatomy and electrical activity and deliver PFA energy to the heart to treat atrial fibrillation. The catheter uses contact sensing to determine which electrodes are in contact with cardiac tissue to help ensure therapy is delivered effectively to the heart.
In the study, patients with paroxysmal AF received pulmonary vein isolation (PVI) treatment in as little as 16 minutes. Patients with persistent AF received PVI treatment, and then the system was used to create a high-definition map of the atrium using 3-D mapping. Using these maps, all of the patients with persistent AF received additional ablation of the posterior wall, and several patients also received a mitral isthmus line.
“It was easy to quickly position the ablation catheter at each pulmonary venous origin, ensure that the electrodes were in good contact with the tissue, and then safely and rapidly isolate the vein by delivering the pulsed field energy,” says Dr. Reddy, the Leona M. and Harry B. Helmsley Charitable Trust Professor of Medicine in Cardiac Electrophysiology at Icahn Mount Sinai. There were no complications.
“PFA rapidly and efficiently isolates pulmonary veins with an important degree of tissue selectivity and a safety profile unachievable in the past for cardiac ablation.”
Vivek Reddy, MD
For decades, thermal energy for catheter ablation of AF has been the accepted standard in cardiac electrophysiology. The downside to thermal energy sources—including radiofrequency, cryothermy, laser and ultrasound—is indiscriminate destruction of all tissue types. As a result, the procedure continues to be associated with infrequent but potentially serious complications such as pulmonary vein stenosis, stroke, phrenic nerve palsy, and atrio-esophageal fistula.
Pulsed field ablation represents a potential paradigm shift in AF ablation, Dr. Reddy says. The modality is a more tissue-specific form of energy delivery than thermal, involving the application of high-intensity direct current pulses of short duration across multiple electrodes without the use of an external patch. “PFA rapidly and efficiently isolates pulmonary veins with an important degree of tissue selectivity and a safety profile unachievable in the past for cardiac ablation,” Dr. Reddy says.
Pulsed field ablation is an adaptation of direct current ablation, which was actually the first energy source used for catheter ablation, in the 1980s to treat cardiac arrhythmias. That ablative methodology was replaced by radiofrequency energy, given its more efficient and precise energy delivery. PFA, with its application of more controlled high-intensity pulses over very short duration, represents the field’s next major advance. During PFA, adjacent myocardial cell membranes are destabilized, resulting in nanoscale pores and increased cell membrane permeability and leakage of cell contents. This phenomenon results in either immediate necrosis or delayed apoptotic cell death.
Beyond safety, the speed with which pulmonary vein isolation can be achieved is a major advantage, shortening procedure times and allowing operators to address other potential sources of AF.
Following this successful first-in-human study, there are active plans to commence a multicenter, multinational FDA trial in early 2023; Mount Sinai will be the lead site for this clinical trial, with Dr. Reddy serving as the overall trial Principal Investigator.
Featured

Vivek Reddy, MD
Director of Cardiac Electrophysiology, and the Leona and Harry B. Helmsley Charitable Trust Professor of Cardiac Electrophysiology