Olpasiran therapy significantly reduced lipoprotein(a) concentrations in patients with established atherosclerotic cardiovascular disease (ASCVD), according to a study led at Mount Sinai Heart by Robert S. Rosenson, MD, Director of Cardiometabolic Disorders, Icahn School of Medicine at Mount Sinai. The study was published in November 2022 in The New England Journal of Medicine.
Higher concentrations of lipoprotein(a), known as Lp(a), have long been associated with ASCVD and aortic stenosis, but no drug therapies that lower this concentration are approved. The phase 2 study found that olpasiran, a small interfering RNA molecule, markedly reduced the concentration of Lp(a) and appeared to be safe. At higher doses, olpasiran therapy reduced the Lp(a) concentration by more than 95 percent compared with a placebo.
The research is part of the OCEAN(a) (Olpasiran Trials of Cardiovascular Events and Lipoprotein(a) Reduction) study funded by Amgen, maker of olpasiran. The phase 2 trial, which had 34 participating sites in seven countries, was designed to evaluate the efficacy and safety of several different doses of olpasiran. The global Principal Investigator of the trial is Michelle L. O'Donoghue, MD, MPH, of Brigham and Women's Hospital in Boston.
Researchers conducted a randomized, double-blind, placebo-controlled, dose-finding trial involving 281 patients with established ASCVD and an Lp(a) concentration of more than 150 nanomoles (nmol) per liter. Patients were randomly assigned to receive a subcutaneous injection of placebo every 12 weeks; 10 mg, 75 mg, or 225 mg of olpasiran every 12 weeks; or 225 mg of olpasiran every 24 weeks, for 48 weeks. The mean age of the patients was 61.9 years, and 32 percent were women. The primary end point was the percent change in the Lp(a) concentration from baseline to week 36. Safety was also assessed.
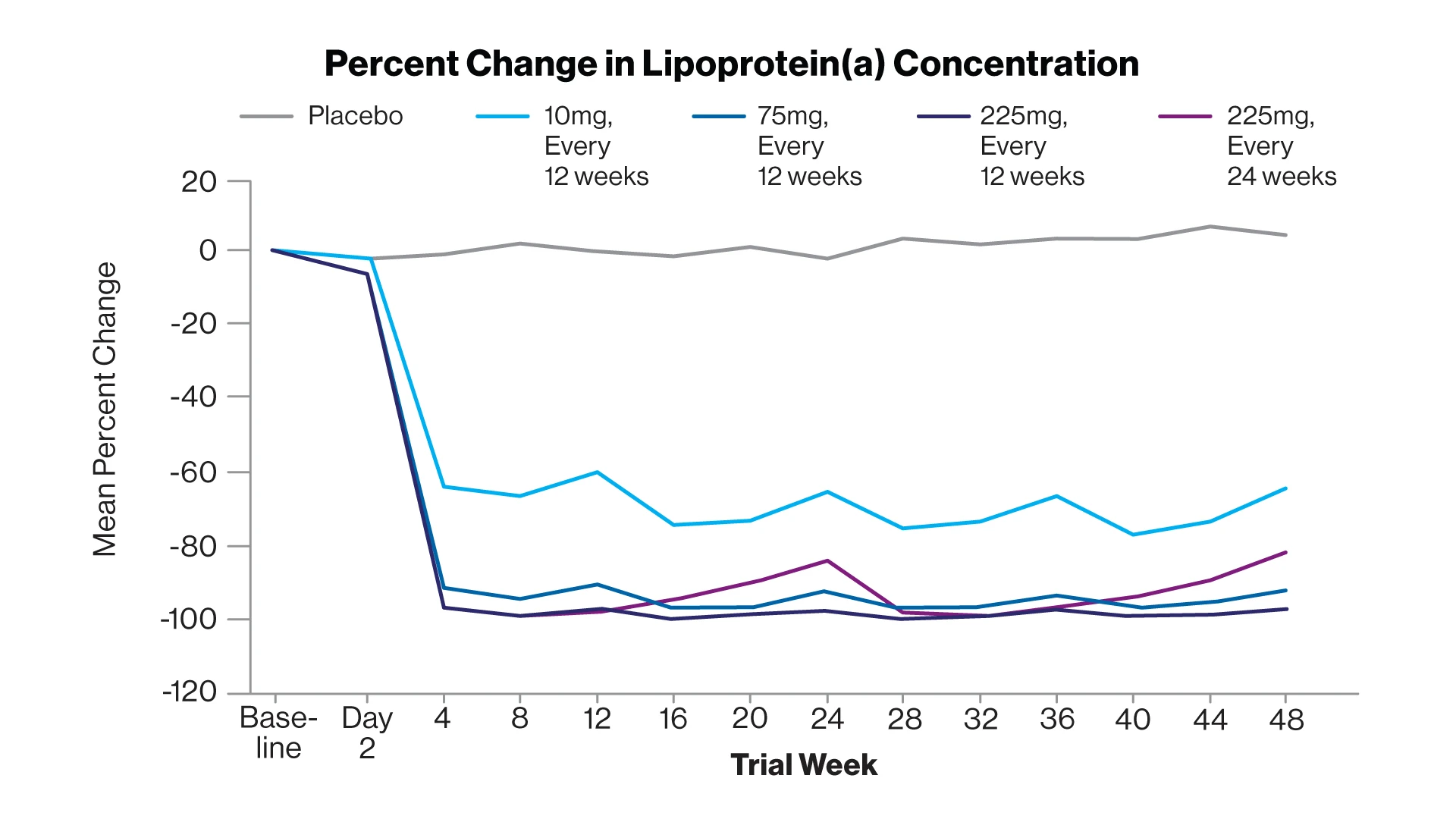
The mean percent change in the lipoprotein(a) concentration over time according to trial group.
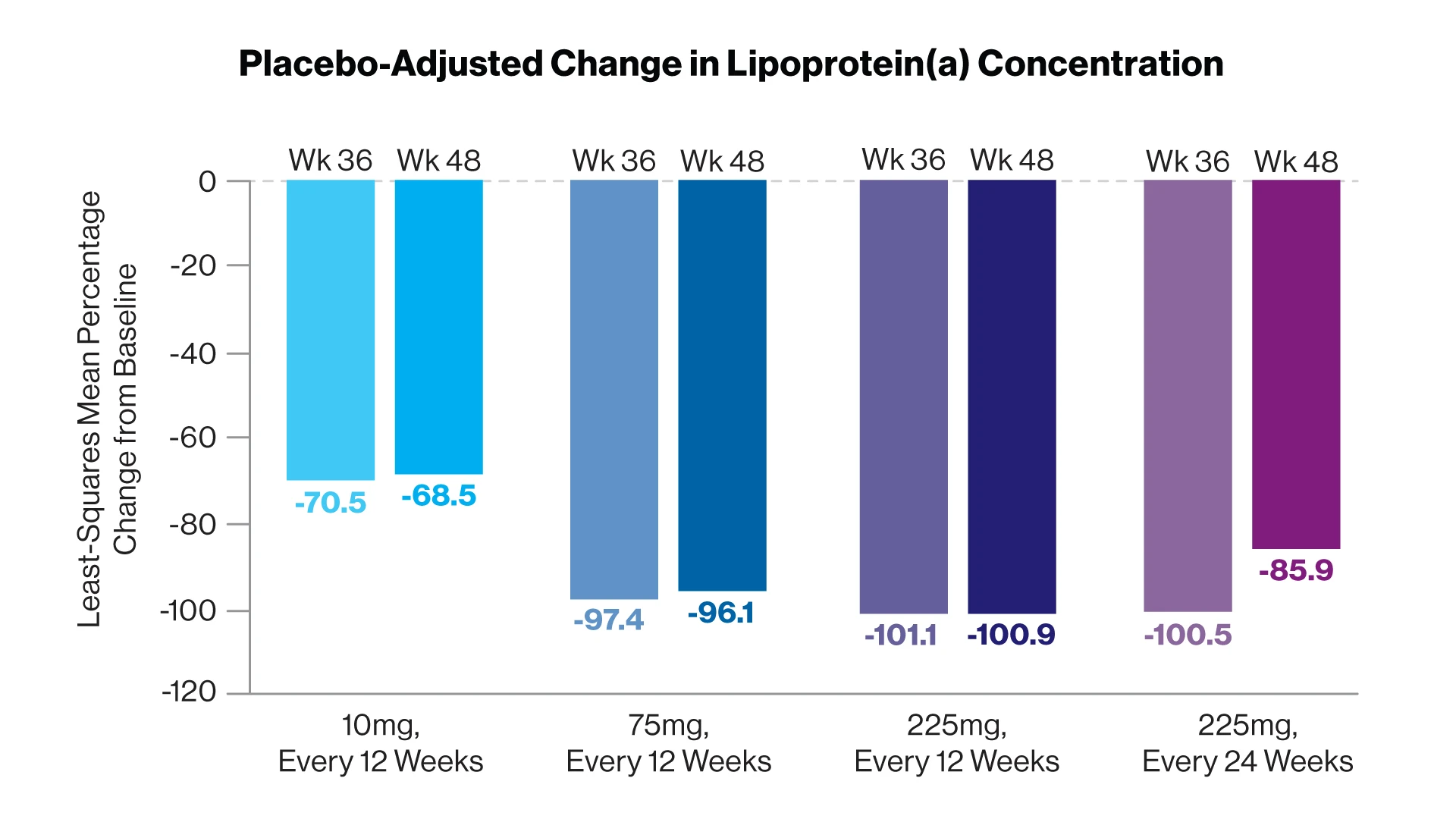
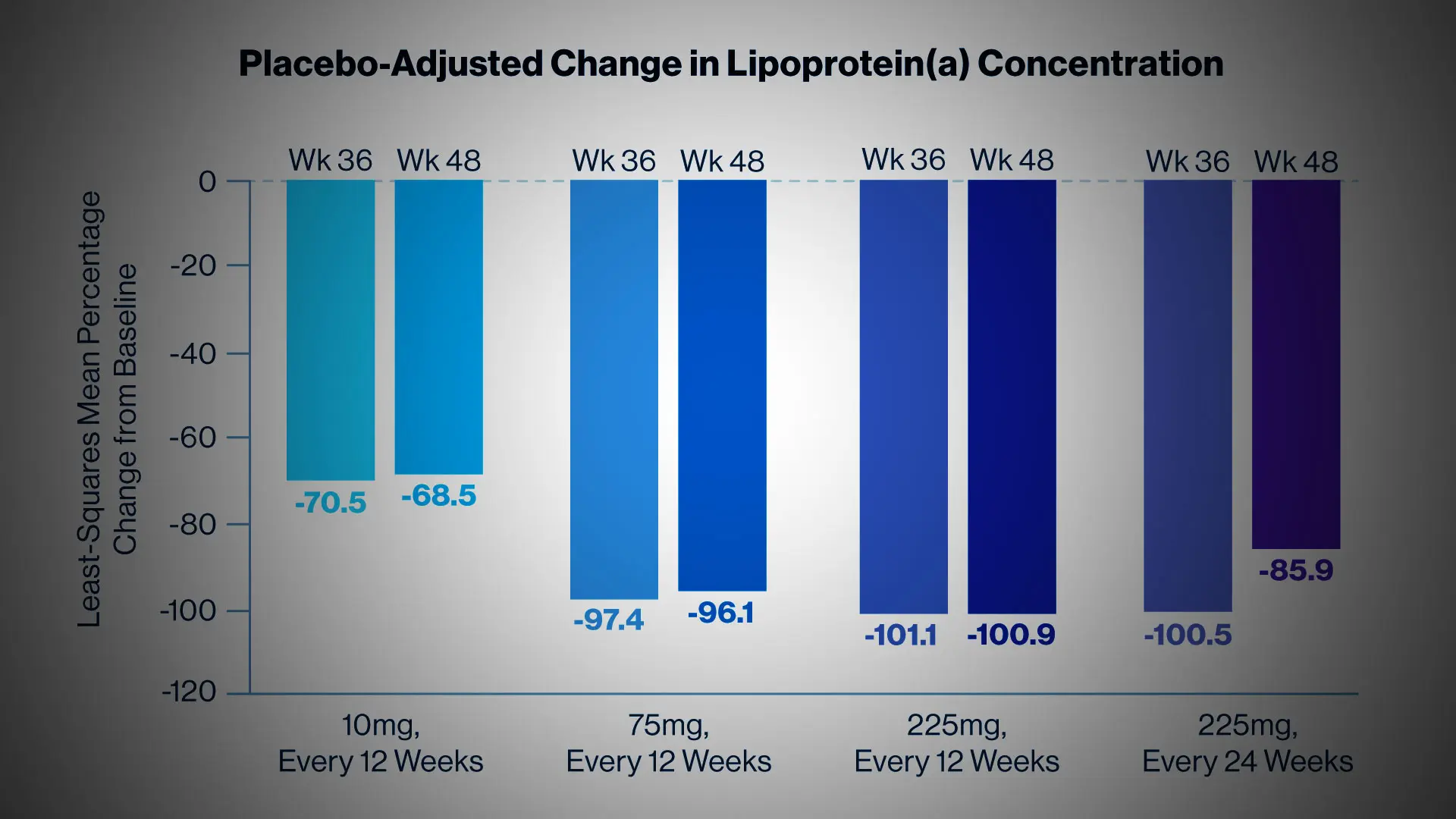
The placebo-adjusted least-squares mean percent change from baseline in the lipoprotein(a) concentration for the four olpasiran dose groups at weeks 36 and 48.
Among the patients, the median concentration of Lp(a) at baseline was 260.3 nmol per liter, and the median concentration of low-density lipoprotein cholesterol was 67.5 mg per deciliter. At baseline, 88 percent of the patients were taking statin therapy, 52 percent were taking ezetimibe, and 23 percent were taking a proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitor.
At 36 weeks, the Lp(a) concentration had increased by a mean of 3.6 percent in the placebo group. In contrast, olpasiran therapy significantly reduced the Lp(a) concentration, resulting in placebo-adjusted mean decreases of 70.5 percent with the 10-mg dose, 97.4 percent with the 75-mg dose, 101.1 percent with the 225-mg dose administered every 12 weeks, and 100.5 percent with the 225-mg dose administered every 24 weeks. The incidence of adverse events was similar across the trial groups, most commonly pain at the injection site.
The team noted areas for further study, for example, how olpasiran therapy might affect the risk of cardiovascular events. In addition, there is no consensus on a threshold between normal and abnormal lipoprotein(a) levels.
An international, multicenter phase 3 OCEAN(a) clinical trial was launched in December 2022, seeking to enroll 6,000 participants. Icahn Mount Sinai is one of 72 sites participating.
The primary objective of the study is to compare the effect of treatment with olpasiran to treatment with a placebo, on the risk for coronary heart disease death, myocardial infarction, or urgent coronary revascularization in participants with ASCVD and elevated Lp(a).
Featured

Robert Rosenson, MD
Professor of Medicine (Cardiology), and Director of Cardiometabolic Disorders