Bivalirudin is a safer and more effective anticoagulant than heparin for treating patients with the most serious type of heart attacks who undergo percutaneous coronary intervention (PCI), and can lower the risk of death or major bleeding by 31 percent, according to a study led by Gregg W. Stone, MD, Professor of Medicine (Cardiology), Icahn School of Medicine at Mount Sinai.
It was the first large-scale clinical trial to compare the two anticoagulants most widely used after PCI, and shows that bivalirudin, given with a two- to four-hour high-dose infusion, significantly reduces death, major bleeding, and thrombosis when compared with heparin. The results were announced in November 2022 in a late-breaking clinical trial presentation at the American Heart Association’s Scientific Sessions, and published in The Lancet.
This work has the potential to change the treatment course for hundreds of thousands of patients across the world who experience a major blockage in a heart artery which causes an ST-segment elevation myocardial infarction (STEMI), says Dr. Stone, co-principal investigator of the study. “Compared with heparin, bivalirudin plus a short infusion substantially improved the likelihood of surviving a STEMI and reduced the two most feared complications—major bleeding and stent thrombosis,” he says.
In the BRIGHT-4 trial, Dr. Stone, along with Yaling Han, MD, PhD, of Shenyang Northern Hospital in China, led a team of researchers in studying 6,106 patients enrolled in the study across 87 sites in China between February 2019 and April 2022. All underwent primary PCI for STEMI treatment, nearly all with a procedure that used the radial artery in the wrist to target the blocked heart artery. Patients were randomized; one group received heparin alone, administered through an IV, and the other group received bivalirudin through an IV, followed by a high-dose IV infusion for two to four hours after the procedure.
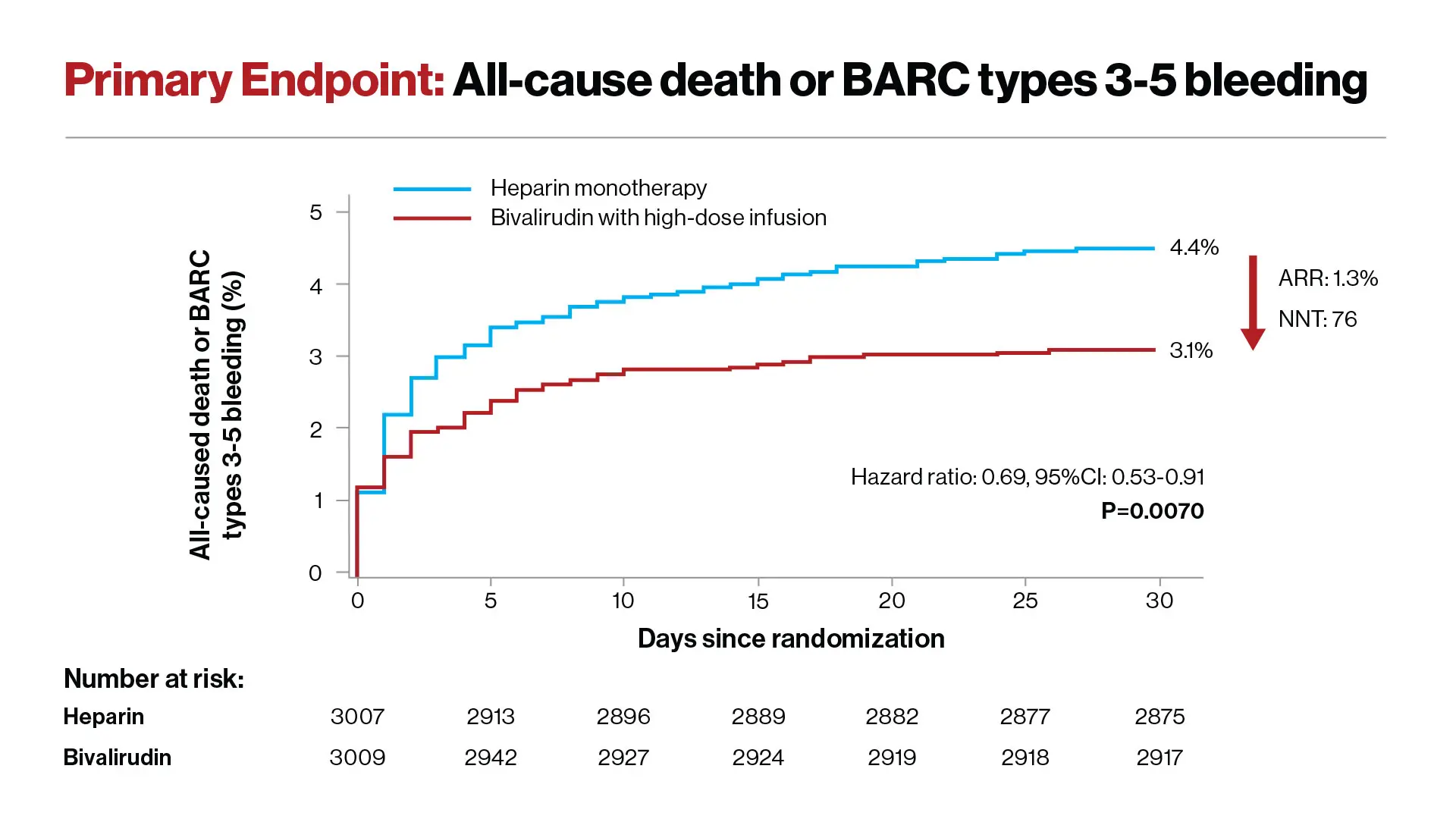
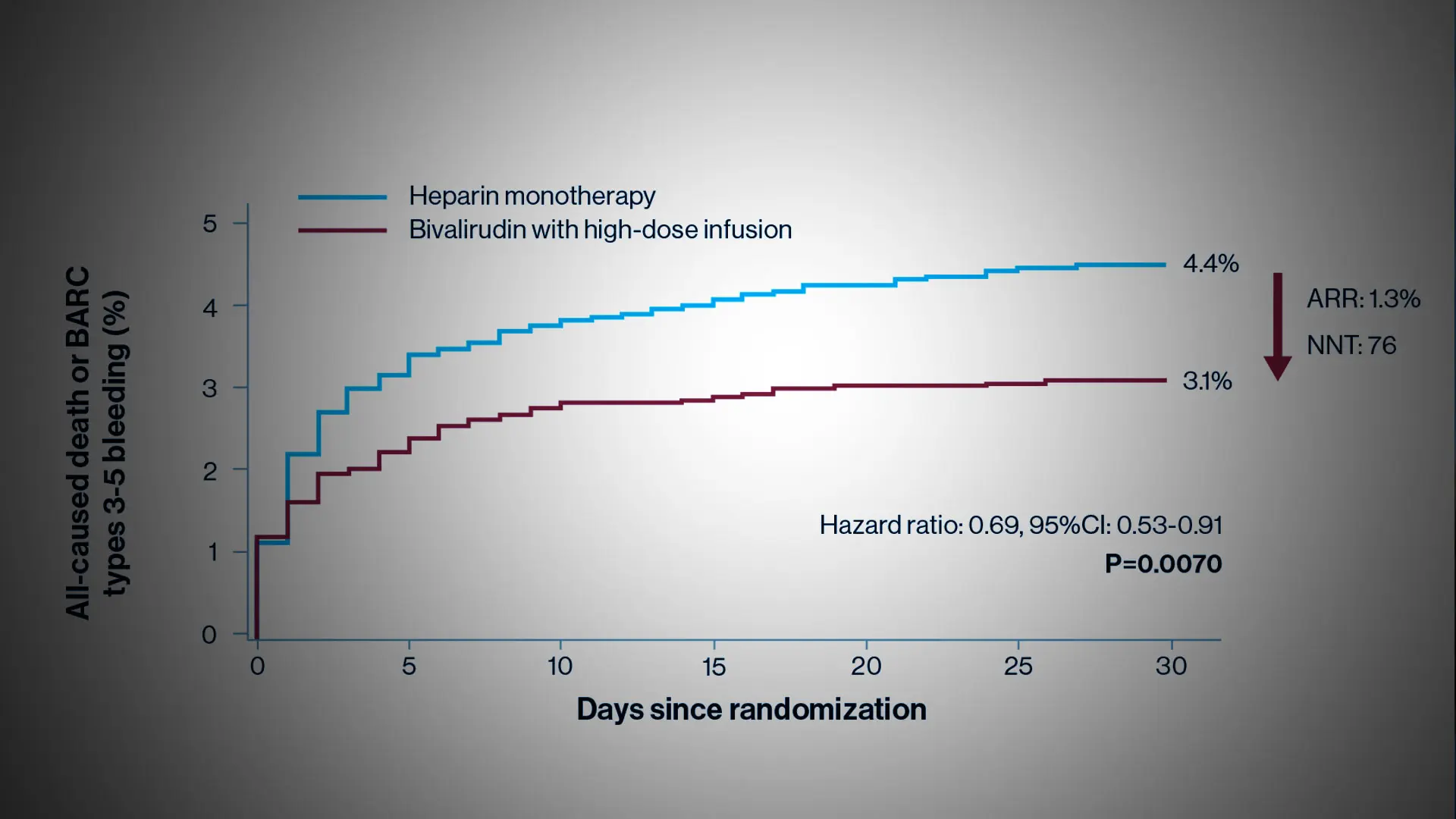
Reduction in the risk of death or major bleeding after primary PCI in patients with STEMI treated with heparin monotherapy (blue curve) or bivalirudin with a post-procedure infusion (red curve). The hazard ratio of 0.69 signifies a 31% reduction in risk. The ARR (absolute risk reduction) was 1.3%.
Investigators followed patients for 30 days following the procedure; the primary endpoint was all-cause mortality or major bleeding. Researchers found that 4.4 percent of patients treated with heparin died or had a major bleed within 30 days, compared to 3.1 percent of patients treated with bivalirudin. Overall, the bivalirudin group had a 31 percent reduction in the rate of death or major bleeding compared with patients in the heparin group—a highly significant reduction.
Then researchers found that deaths were reduced from 3.9 percent in heparin-treated patients to 3.0 percent in bivalirudin-treated patients. Severe bleeding also was reduced from 0.8 percent in the heparin group to 0.2 percent in the bivalirudin group. Both of these differences were statistically significant.
They also analyzed the rate of stent thrombosis, which can lead to a second heart attack or death. This occurred in 0.4 percent of patients in the bivalirudin group, compared with 1.1 percent in the heparin group, again a statistically significant reduction.
“These results are dramatic,” Dr. Stone said. “The simple decision to use bivalirudin during primary PCI in patients with heart attacks, which is now generic and thus inexpensive, can save hundreds of thousands of lives per year and prevent major bleeding and stent thrombosis compared with heparin.”
BRIGHT-4 was an investigator-sponsored and organized trial. It was funded by the Chinese Society of Cardiology Foundation with a research grant from Jiangsu Hengrui Pharmaceuticals Co., Ltd.
Featured

Gregg W. Stone, MD
Professor of Medicine (Cardiology), and Population Health Science and Policy