A study showing the safety and effectiveness of a leadless cardiac pacemaker for right
ventricular pacing has set the stage for research into a novel dual-chamber device that would
serve a significantly broader population of patients with abnormal heart rhythms. A global
clinical trial of the first-in-human dual chamber system is now enrolling, with Mount Sinai as a
key investigative site.
“The single-chamber pacemaker is a major advance over the conventional transvenous
pacemaker, particularly when a physician is concerned about invasive implantation surgery or
when a patient’s therapy may change in the future and retrievability is a vital design
consideration,” says Vivek Reddy, MD, Professor of Medicine (Cardiology), and first author of
the study published in November 2021 in the Journal of the American College of Cardiology:
Clinical Electrophysiology. “But single-chamber pacing is relevant to only 10 percent to 15
percent of patients worldwide requiring pacemakers, which is why the dual-chamber system
represents such promising new technology.”
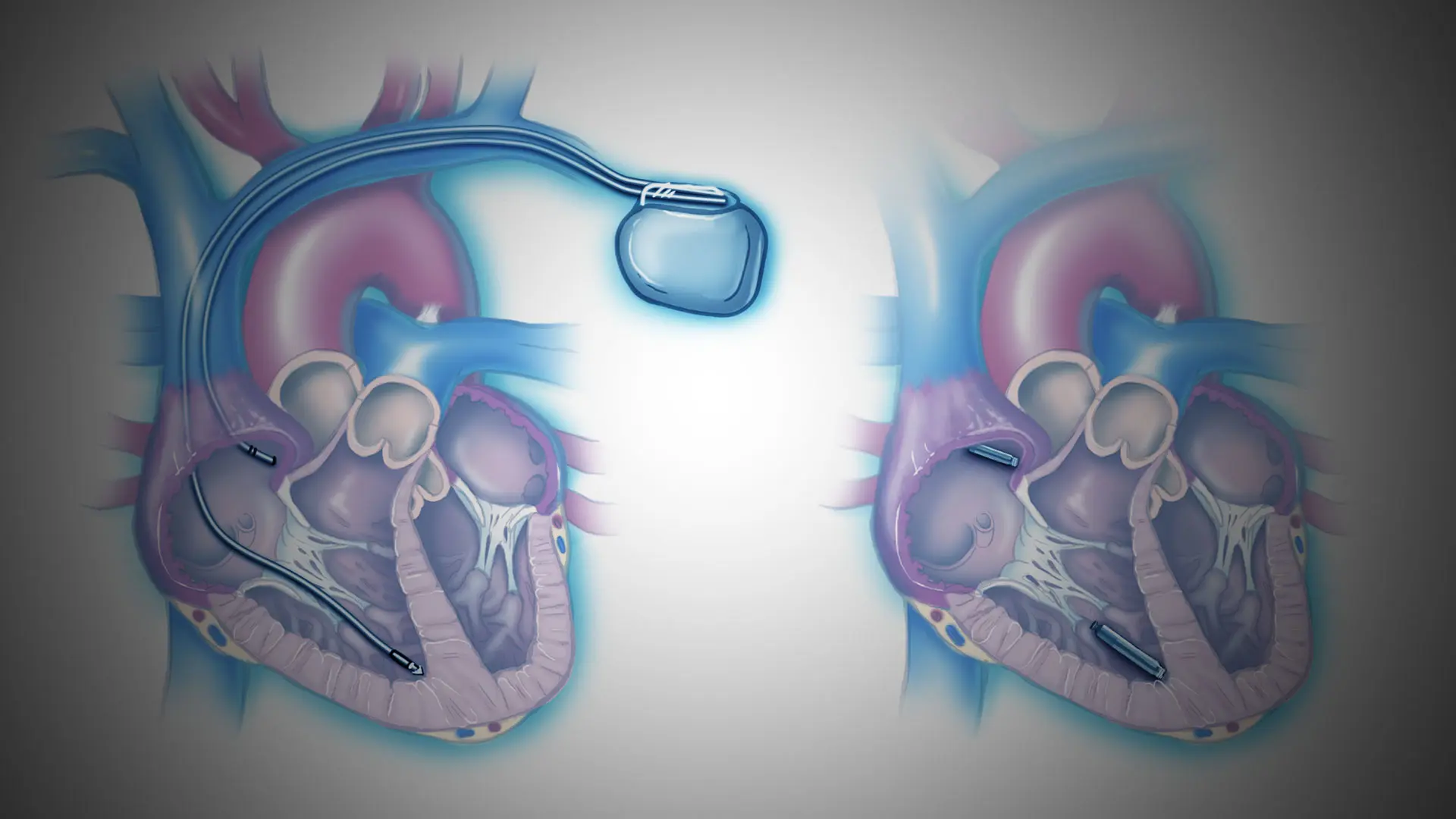
A dual-chamber leadless pacemaker provides both atrial and ventricular bradycardia therapy
while eliminating the long-term complications associated with the surgical pocket and
transvenous atrial and ventricular leads. The LEADLESS II-Phase 2 trial—which enrolled 200
patients with atrial fibrillation and atrioventricular block across 43 sites in the United States,
Canada, and Europe—evaluated the single-chamber Aveir leadless pacemaker (Abbott). An important feature
of this single-chamber device is that it provides an expandable platform to support a fully
leadless dual-chamber pacing device, once that is approved. Operationally, this would allow an Aveir device in the ventricle and a secondary device in the atrium to seamlessly communicate.
Indeed, the LEADLESS II-Phase 2 study set the stage for the trial of the dual-chamber Aveir leadless pacing system—the Aveir DR i2i Study—which commenced in February 2022. The first patients in the world were treated by Dr. Reddy along with his colleague, Petr Neuzil, MD, Director of Cardiology, Homolka Hospital, Prague, Czech Republic.
The first leadless cardiac pacemaker, the Nanostim, was introduced by St. Jude Medical in 2013, but was removed from the market because of premature battery depletion. Another single-chamber leadless device, the Micra Transcatheter Pacing System by Medtronic, was approved in the United States in 2016, and remains the only leadless pacemaker now available to patients.
“We believe that, once approved, the device will become a critical tool in the armamentarium of physicians who care for millions of people with abnormal heart rhythms.”
- Vivek Reddy, MD
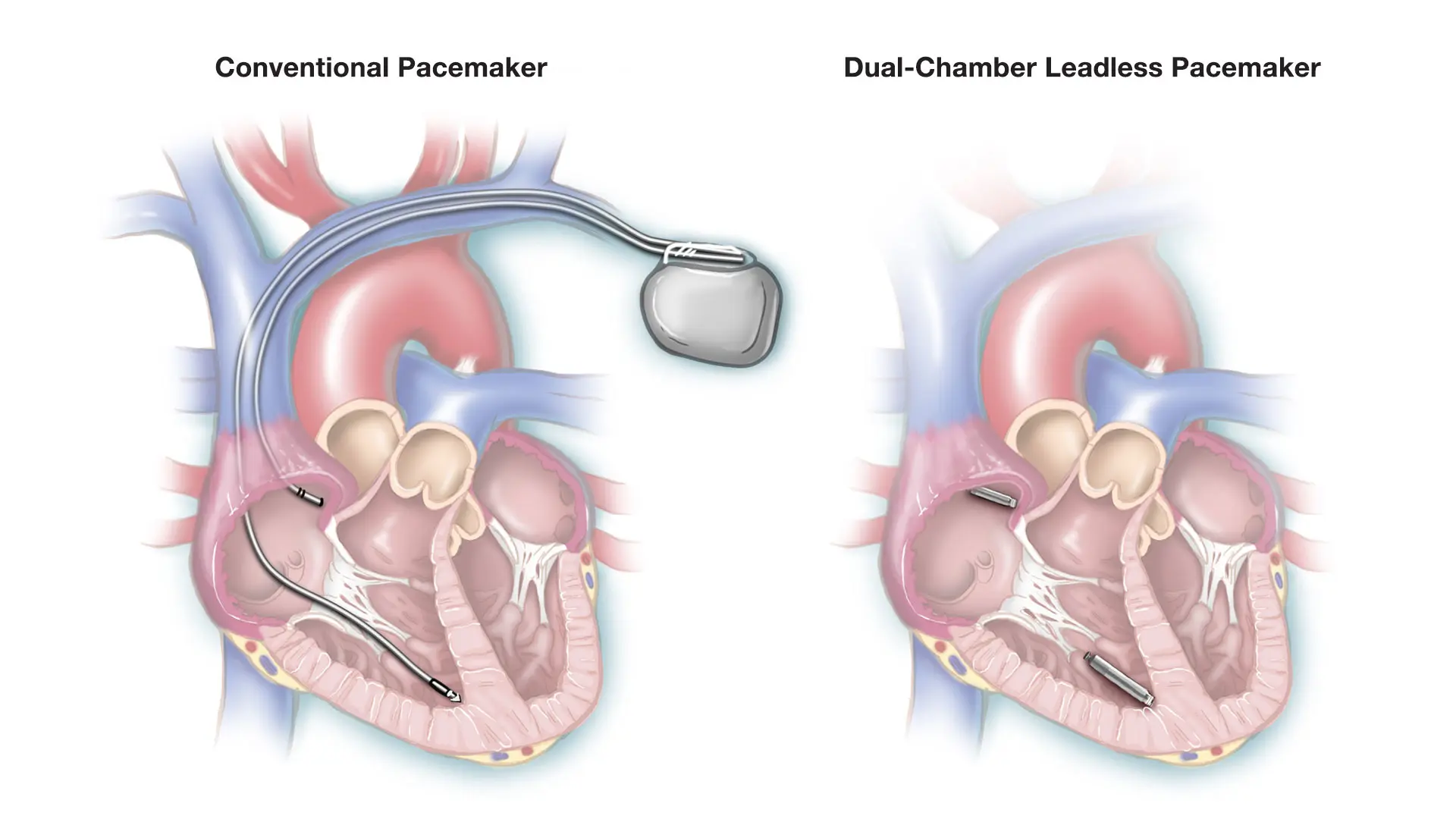
A traditional system with leads and pacemaker generator, left, and the leadless system with two devices that communicate with each other.
The LEADLESS II-Phase 2 study could potentially change that landscape. It is the first-in-human trial of a redesigned cardiac pacemaker with significant improvements over the original technology. These include the use of lithium carbon-monofluoride chemistry increasing the battery life by 1.1 years, to 10.4 years (potentially lasting about 25 years for patients requiring infrequent pacing); a modified docking button enabling retrievability; an improved delivery system with ergonomic design; and a new application-specific integrated circuit (ASIC) chip to provide an expandable platform to later support dual-chamber pacing.
Results of the LEADLESS II-Phase 2 study were presented in November 2021 at the annual
scientific sessions of the Asia Pacific Heart Rhythm Society, and also submitted to the U.S. Food and Drug Administration for investigative review.
The study met all of its clinical safety and efficacy endpoints. The primary safety endpoint was
defined as freedom from serious adverse device effects through six weeks of follow-up. Efficacy was measured by acceptable pacing capture threshold (≤ 2.0 V at 0.4 msec) and sensing amplitude (R wave ≥ 5.0 mV), or a value equal to or greater than the value at implantation). The device was successfully implanted in 98 percent of patients, of which 83 percent did not require repositioning.
“Not only does the redesigned system have a greatly improved battery life, but it’s the only
leadless pacemaker in the world designed to be retrieved when the device needs to be
replaced, or a patient’s therapy needs to be changed,” says Dr. Reddy, Director of Cardiac
Arrhythmia Service at Mount Sinai. “We believe that, once approved, the device will become a
critical tool in the armamentarium of physicians who care for millions of people with
abnormal heart rhythms.”
Vivek Reddy, MD, has reported serving as a consultant for Abbott, the manufacturer of the Aveir leadless pacemaker technology.
Featured

Vivek Reddy, MD
Director of Cardiac Electrophysiology, and the Leona and Harry B. Helmsley Charitable Trust Professor of Cardiac Electrophysiology