Mount Sinai’s groundbreaking global TWILIGHT study—which suggested that selected patients do not need long-term aspirin after percutaneous coronary intervention (PCI)—has resonated across cardiology, supported by follow-up studies and informing new guidelines from the European Society of Cardiology.
“TWILIGHT opened the door to a new and innovative way of caring for heart patients at risk for both bleeding and ischemic complications,” says Roxana Mehran, MD, the study’s global principal investigator, and Director of the Center for Interventional Cardiovascular Research and Clinical Trials at Mount Sinai Heart. “We showed that in some patients less is more, and the fact that principle is now starting to influence practice guidelines around the world demonstrates the importance of bleeding-avoidance strategies for targeted care of patients after coronary intervention.”
The TWILIGHT study, published in November 2019 in The New England Journal of Medicine,
addressed long-standing questions about the treatment of high-risk patients after PCI. “The same patients who are at high risk for ischemic events—such as heart attack or stroke caused by blocked blood vessels—are also at high risk for bleeding events,” Dr. Mehran says. “You have to find the right balance in therapies.”
The standard of care for these patients has long been dual antiplatelet therapy (DAPT), combining aspirin with a potent anti-clotting medication, such as ticagrelor, to lower the risk of heart attack. The TWILIGHT study of more than 9,000 high-risk cardiac patients following PCI found that withdrawing aspirin after three months of DAPT reduced bleeding by 44 percent, and resulted in no differences in the risk of heart attack, death, or stroke between the two groups, suggesting that discontinuation of aspirin does not compromise safety. The results were consistent in both men and women, and in patients older and younger than 65.
These findings were used as evidence for an important addition in the European Society of Cardiology’s 2020 guidelines, published in the European Heart Journal, adding a class 2a recommendation for ticagrelor monotherapy in the management of selected patients with acute coronary syndromes (ACS) without persistent ST-segment elevations.
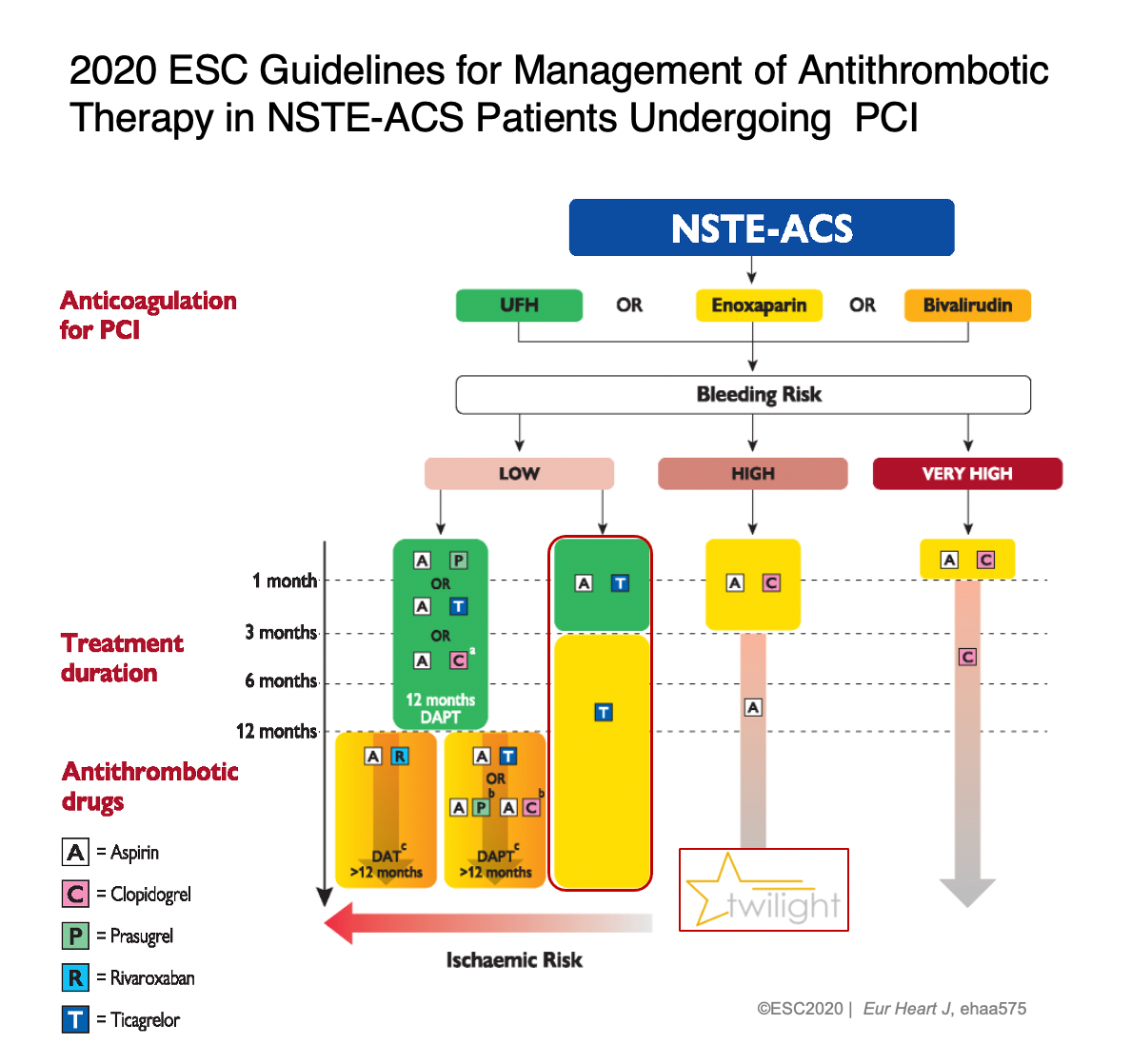
The European Society of Cardiology’s 2020 guidelines added a class 2a recommendation for
ticagrelor monotherapy in the management of selected patients with acute coronary syndromes (ACS) without persistent ST-segment elevations.
The same journal in October 2020 published results of a pre-specified sub-group of patients from the TWILIGHT study on those who presented with non-ST-segment elevation ACS. Researchers in this investigation, led by Usman Baber, MD, Assistant Professor of Medicine (Cardiology), Icahn School of Medicine at Mount Sinai, found that bleeding events in patients on ticagrelor therapy alone were significantly reduced without any increases in ischemic risk compared to those who continued aspirin therapy in combination with ticagrelor.
Another study focused on the diabetic population enrolled in the TWILIGHT trial. It was published in May 2020 in the Journal of American Cardiology and similarly found that the effect of ticagrelor monotherapy in reducing the risk of clinically relevant bleeding without any increase in ischemic events was consistent among patients with or without diabetes mellitus undergoing PCI.
“The findings from TWILIGHT are not only gaining traction with cardiologists everywhere, but allowing them to exercise greater control over bleeding and ischemic complications by eliminating long-term aspirin use in their patients,” says Dr. Mehran, the Mount Sinai Professor in Cardiovascular Clinical Research and Outcomes, Icahn School of Medicine at Mount Sinai. “We’re continuing to actively evaluate and provide data for many other subsets of heart patients who could benefit from this simpler but highly effective approach.”
The drug ticagrelor is made by AstraZeneca, which provided Mount Sinai with an unrestricted grant to perform the investigator-initiated TWILIGHT study. Dr. Baber has received consulting fees from AstraZeneca in the past.
Featured

Roxana Mehran, MD
Mount Sinai Professor in Cardiovascular Clinical Research and Outcomes, and Director of the Center for Interventional Cardiovascular Research

Usman Baber, MD
Assistant Professor of Medicine (Cardiology), Icahn School of Medicine at Mount Sinai