Building on treatment protocols developed at the height of the COVID-19 pandemic, Mount Sinai Heart has launched the global FREEDOM COVID-19 Anticoagulation Trial to determine the most effective dosage and regimen of anticoagulant therapy in improving the survival rate of hospitalized COVID-19 patients.
“While vaccines are being developed and clinical trials are now underway, there is an urgent need to determine how to best treat the next hundreds of thousands of COVID-19 patients around the world,” says Valentin Fuster, MD, PhD, Director of Mount Sinai Heart and Physician-in-Chief of The Mount Sinai Hospital, and principal investigator of the FREEDOM COVID-19 Trial. “This is a coordinated international effort, launched as an investigator-led trial to speed up the process.”
In March 2020, during the early days of the pandemic, Dr. Fuster closely observed patients with blood clots in their legs who had been admitted with COVID-19. After hearing from colleagues in China of other cases of small, pervasive, and unusual clotting that had triggered myocardial infarctions, strokes, and pulmonary embolisms, he initiated decisive action. “We became one of the first medical centers in the world to treat all COVID-19 patients with anticoagulant medications,” says Dr. Fuster, a pioneer in the study of atherothrombotic disease. “It was a decision that we believe saved many lives.”
That early protocol—based largely on intuition, Dr. Fuster says—led to groundbreaking research and insights by Mount Sinai into the role of anticoagulation in the management of COVID-19-infected patients. That work includes a study published in August 2020 in the Journal of the American College of Cardiology that showed a 50 percent higher chance of survival compared to patients given no anticoagulant. The analysis was conducted by the Mount Sinai COVID Informatics Center.
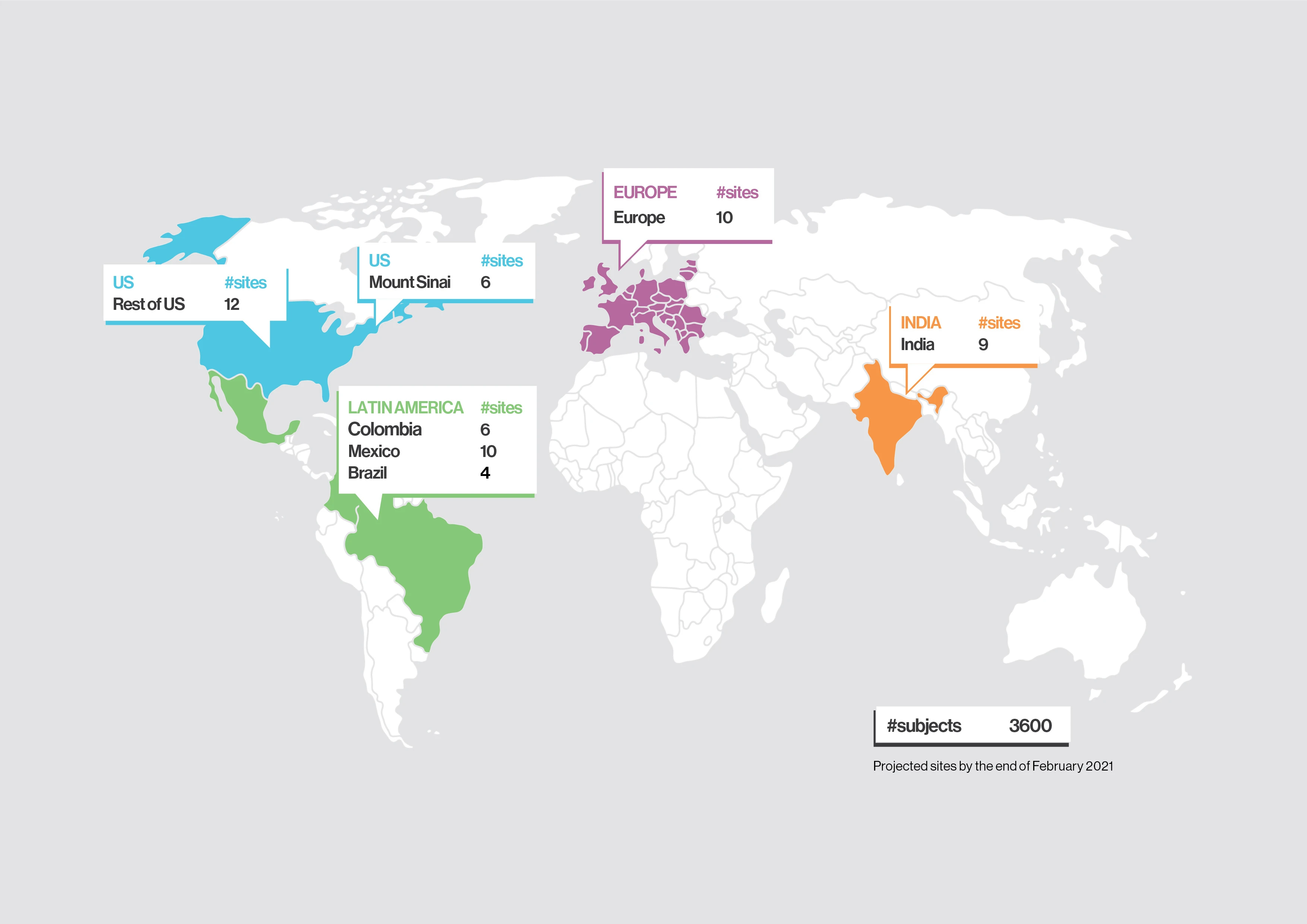
Dr. Fuster says, “While our study was observational, it helped us design the large-scale FREEDOM COVID Anticoagulation Trial in partnership with more than 70 sites in nine countries in order to reach 3,600 patients.” The trial is a prospective study that will be randomized to three different anticoagulation regimens: 1) prophylactic enoxaparin (40 mg), 2) full-dose enoxaparin (1 mg/kg), and 3) apixaban (5 mg). It is designed to study the effectiveness and safety of enoxaparin and apixaban in patients age 18 and older who have been hospitalized with confirmed COVID-19, but not in an ICU or intubated. The trial is currently enrolling and set for completion in June 2021.
Valentin Fuster, MD, PhD, describes the development of anticoagulant protocols to treat patients hospitalized with COVID-19, and the launching of the FREEDOM COVID trial, at the Mount Sinai Clinical Intelligence Center COVID-19 Research Symposium in November 2020. (Viewing time: 7 min)
More specifically, the FREEDOM trial is based on months of clinical observation and pathology laboratory work conducted as deaths from the COVID-19 pandemic mounted globally in spring 2020 and thromboembolism emerged as an important disease manifestation. Autopsy studies at Mount Sinai demonstrated a high incidence of macrothrombi and microthrombi, prompting the suggestion that in-hospital use of blood thinners could be beneficial to COVID-19 patients. To help clarify the pivotal questions of anticoagulant choice, dosing, and treatment duration, Mount Sinai began an observational study in May 2020 of 4,389 COVID-19-positive patients who were admitted to five hospitals in the Mount Sinai Health System.
“In this observational study, anticoagulation was associated with improved outcomes and bleeding rates appeared to be low,” says corresponding author Anuradha Lala, MD, Assistant Professor of Medicine (Cardiology), and Director of Heart Failure Research at the Icahn School of Medicine at Mount Sinai. “As a clinician who has treated COVID-19 patients on the front lines, the importance of having answers as to what the best treatment for these patients entails is immeasurable. Ultimately, clinical trials are what will inform those answers.”
Researchers looked at six different anticoagulant regimens, including oral and intravenous dosing within both therapeutic and prophylactic groups. They observed that subcutaneous low-molecular weight heparin from both groups, and oral apixaban from the therapeutic group, could lead to better outcomes. Both therapeutic and prophylactic doses of anticoagulants were associated with significantly improved in-hospital survival, and with a 30 percent reduced risk of admission to an ICU or intubation. The researchers used a hazard score to estimate risk of death, which took relevant risk factors into account before evaluating the effectiveness of anticoagulation, including age, ethnicity, pre-existing conditions, and whether the patient was already on blood thinners.
Separately, the researchers looked at autopsy results of 26 COVID-19 patients and found that 11 of them (42 percent) had blood clots—pulmonary, brain, and/or heart—that were never suspected in the clinical setting. These findings suggest that treating COVID-19 patients with anticoagulants as a preventive measure may be associated with improved survival.
Researchers were further encouraged by the finding that overall rates of major bleeding were low (3 percent or less), though slightly higher in the therapeutic group compared to the prophylactic and no-blood-thinner groups. This suggested to the team that clinicians should evaluate COVID-19 patients on an individual basis, weighing the risks and benefits of anticoagulant therapy.
"These observational analyses were done with the highest level of statistical rigor and provide important insights into the association of anticoagulation with critical in-hospital outcomes of mortality and intubation," says first author Girish Nadkarni, MD, Co-Founder and Co-Director of the Mount Sinai COVID Informatics Center, and Clinical Director of the Hasso Plattner Institute for Digital Health at Mount Sinai.
The study also provided a strong rationale for the FREEDOM Trial, says co-author Zahi A. Fayad, PhD, Professor of Medicine (Cardiology), and Diagnostic, Molecular and Interventional Radiology, and Director of Mount Sinai’s BioMedical Engineering and Imaging Institute. “This work highlights the need to better understand the disease from a diagnostic and therapeutic point of view and the importance of conducting properly designed diagnostic and interventional studies.”
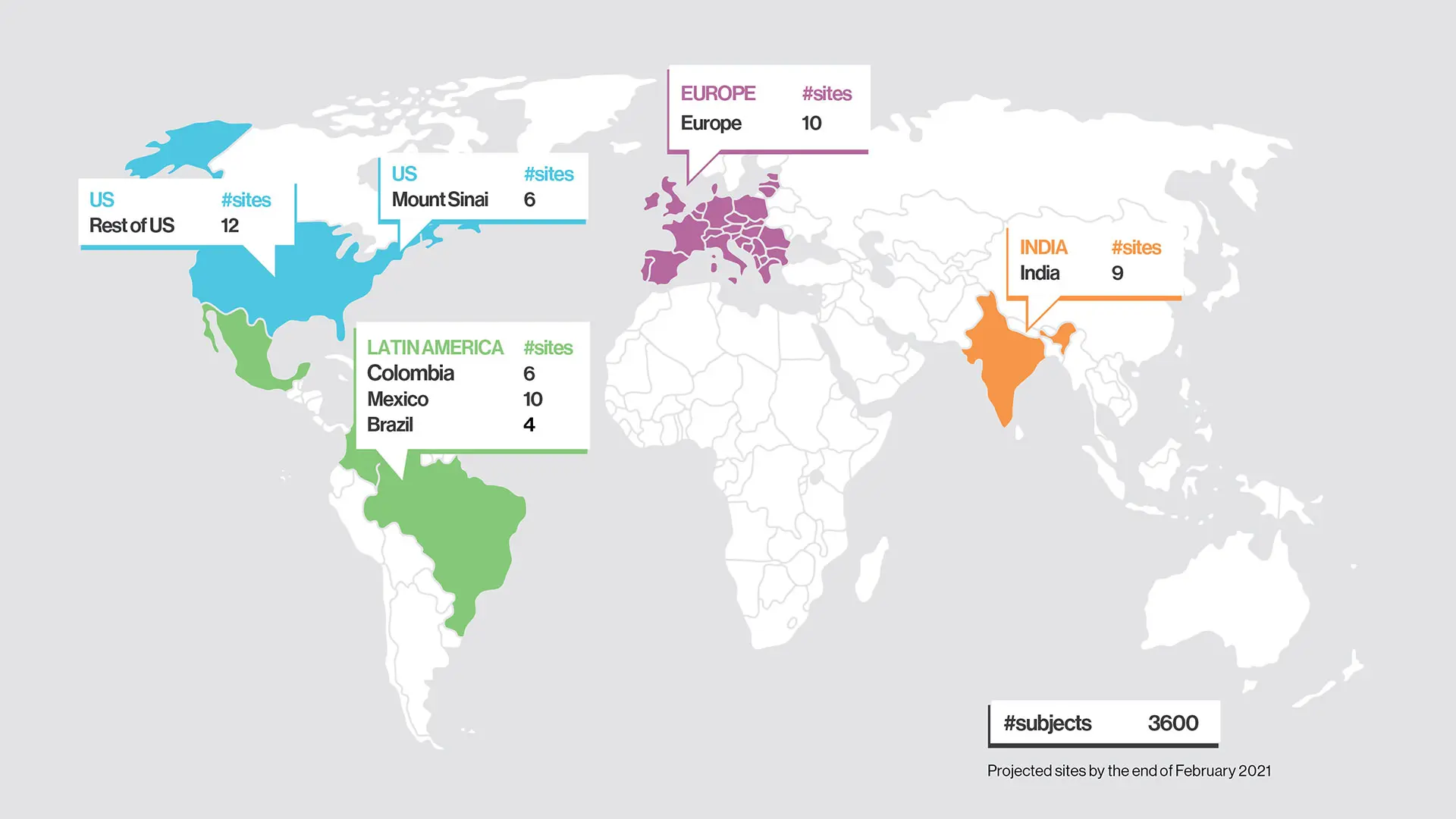
FREEDOM COVID-19 Anticoagulation Trial sites, as of February 2021.
Featured

Girish N. Nadkarni, MD, MPH
Chair of the Windreich Department of Artificial Intelligence and Human Health, Director of the Hasso Plattner Institute for Digital Health, Chief AI Officer, Mount Sinai Health System

Lili Chan, MD
Assistant Professor of Medicine (Nephrology)